Oxaliplatin induces different cellular and molecular chemoresistance patterns in colorectal cancer cell lines of identical origins
- PMID: 23865481
- PMCID: PMC3776436
- DOI: 10.1186/1471-2164-14-480
Oxaliplatin induces different cellular and molecular chemoresistance patterns in colorectal cancer cell lines of identical origins
Abstract
Background: Cancer cells frequently adopt cellular and molecular alterations and acquire resistance to cytostatic drugs. Chemotherapy with oxaliplatin is among the leading treatments for colorectal cancer with a response rate of 50%, inducing intrastrand cross-links on the DNA. Despite of this drug's efficiency, resistance develops in nearly all metastatic patients. Chemoresistance being of crucial importance for the drug's clinical efficiency this study aimed to contribute to the identification and description of some cellular and molecular alterations induced by prolonged oxaliplatin therapy. Resistance to oxaliplatin was induced in Colo320 (Colo320R) and HT-29 (HT-29R) colorectal adenocarcinoma cell lines by exposing the cells to increasing concentrations of the drug. Alterations in morphology, cytotoxicity, DNA cross-links formation and gene expression profiles were assessed in the parental and resistant variants with microscopy, MTT, alkaline comet and pangenomic microarray assays, respectively.
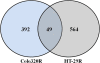
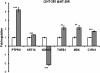
Results: Morphology analysis revealed epithelial-to-mesenchymal transition in the resistant vs parental cells suggesting alterations of the cells' adhesion complexes, through which they acquire increased invasiveness and adherence. Cytotoxicity measurements demonstrated resistance to oxaliplatin in both cell lines; Colo320 being more sensitive than HT-29 to this drug (P < 0.001). The treatment with oxaliplatin caused major DNA cross-links in both parental cell lines; in Colo320R small amounts of DNA cross-links were still detectable, while in HT-29R not. We identified 441 differentially expressed genes in Colo320R and 613 in HT-29R as compared to their parental counterparts (at least 1.5 -fold up- or down- regulation, p < 0.05). More disrupted functions and pathways were detected in HT-29R cell line than in Colo320R, involving genes responsible for apoptosis inhibition, cellular proliferation and epithelial-to-mesenchymal transition. Several upstream regulators were detected as activated in HT-29R cell line, but not in Colo320R.
Conclusions: Our findings revealed a more resistant phenotype in HT-29R as compared to Colo320R and different cellular and molecular chemoresistance patterns induced by prolonged treatment with oxaliplatin in cell lines with identical origins (colorectal adenocarcinomas).
Figures

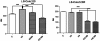
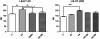

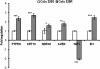
Similar articles
-
Assessment of cytotoxicity, apoptosis and DNA damages in Colo320 colorectal cancer cells selected for oxaliplatin resistance.Cell Biochem Funct. 2011 Jul;29(5):351-5. doi: 10.1002/cbf.1754. Epub 2011 Apr 13. Cell Biochem Funct. 2011. PMID: 21491469
-
Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines.Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4147-53. doi: 10.1158/1078-0432.CCR-06-0038. Clin Cancer Res. 2006. PMID: 16857785
-
Superior cytotoxicity and DNA cross-link induction by oxaliplatin versus cisplatin at lower cellular uptake in colorectal cancer cell lines.Anticancer Drugs. 2012 Nov;23(10):1032-8. doi: 10.1097/CAD.0b013e328355076f. Anticancer Drugs. 2012. PMID: 22614106
-
The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer.Nutrients. 2023 Nov 7;15(22):4705. doi: 10.3390/nu15224705. Nutrients. 2023. PMID: 38004099 Free PMC article.
-
The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer.Theranostics. 2020 Jan 12;10(5):1981-1996. doi: 10.7150/thno.37621. eCollection 2020. Theranostics. 2020. PMID: 32104496 Free PMC article.
Cited by
-
Connexin 32 downregulation is critical for chemoresistance in oxaliplatin-resistant HCC cells associated with EMT.Cancer Manag Res. 2019 May 31;11:5133-5146. doi: 10.2147/CMAR.S203656. eCollection 2019. Cancer Manag Res. 2019. PMID: 31213923 Free PMC article.
-
Licochalcone B Induces ROS-Dependent Apoptosis in Oxaliplatin-Resistant Colorectal Cancer Cells via p38/JNK MAPK Signaling.Antioxidants (Basel). 2023 Mar 7;12(3):656. doi: 10.3390/antiox12030656. Antioxidants (Basel). 2023. PMID: 36978904 Free PMC article.
-
Computational Tactics for Precision Cancer Network Biology.Int J Mol Sci. 2022 Nov 19;23(22):14398. doi: 10.3390/ijms232214398. Int J Mol Sci. 2022. PMID: 36430875 Free PMC article. Review.
-
Novel Palladium(II) Complexes that Influence Prominin-1/CD133 Expression and Stem Cell Factor Release in Tumor Cells.Molecules. 2017 Mar 30;22(4):561. doi: 10.3390/molecules22040561. Molecules. 2017. PMID: 28358339 Free PMC article.
-
Microarray Analysis of Gene Expression Involved in Butyrate-Resistant Colorectal Carcinoma HCT116 Cells.Asian Pac J Cancer Prev. 2020 Jun 1;21(6):1739-1746. doi: 10.31557/APJCP.2020.21.6.1739. Asian Pac J Cancer Prev. 2020. PMID: 32592372 Free PMC article.
References
-
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. - PubMed
-
- Woynarowski JM, Faivre S, Herzig MC, Arnett B, Chapman WG, Trevino AV, Raymond E, Chaney SG, Vaisman A, Varchenko M, Juniewicz PE. Oxaliplatin-induced damage of cellular DNA. Mol Pharmacol. 2000;58(5):920–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

