Rho GTPase-independent regulation of mitotic progression by the RhoGEF Net1
- PMID: 23864709
- PMCID: PMC3756918
- DOI: 10.1091/mbc.E13-01-0061
Rho GTPase-independent regulation of mitotic progression by the RhoGEF Net1
Abstract
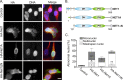
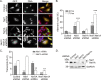
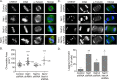
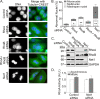
Neuroepithelial transforming gene 1 (Net1) is a RhoA-subfamily-specific guanine nucleotide exchange factor that is overexpressed in multiple human cancers and is required for proliferation. Molecular mechanisms underlying its role in cell proliferation are unknown. Here we show that overexpression or knockdown of Net1 causes mitotic defects. Net1 is required for chromosome congression during metaphase and generation of stable kinetochore microtubule attachments. Accordingly, inhibition of Net1 expression results in spindle assembly checkpoint activation. The ability of Net1 to control mitosis is independent of RhoA or RhoB activation, as knockdown of either GTPase does not phenocopy effects of Net1 knockdown on nuclear morphology, and effects of Net1 knockdown are effectively rescued by expression of catalytically inactive Net1. We also observe that Net1 expression is required for centrosomal activation of p21-activated kinase and its downstream kinase Aurora A, which are critical regulators of centrosome maturation and spindle assembly. These results identify Net1 as a novel regulator of mitosis and indicate that altered expression of Net1, as occurs in human cancers, may adversely affect genomic stability.
Figures


Similar articles
-
Rho GTPase independent regulation of ATM activation and cell survival by the RhoGEF Net1A.Cell Cycle. 2014;13(17):2765-72. doi: 10.4161/15384101.2015.945865. Cell Cycle. 2014. PMID: 25486363 Free PMC article.
-
Cdk1 phosphorylation negatively regulates the activity of Net1 towards RhoA during mitosis.Cell Signal. 2021 Apr;80:109926. doi: 10.1016/j.cellsig.2021.109926. Epub 2021 Jan 17. Cell Signal. 2021. PMID: 33465404 Free PMC article.
-
The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals.PLoS One. 2011 Feb 24;6(2):e17380. doi: 10.1371/journal.pone.0017380. PLoS One. 2011. PMID: 21390328 Free PMC article.
-
Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells.Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24988197 Free PMC article. Review.
-
Aurora kinases link chromosome segregation and cell division to cancer susceptibility.Curr Opin Genet Dev. 2004 Feb;14(1):29-36. doi: 10.1016/j.gde.2003.11.006. Curr Opin Genet Dev. 2004. PMID: 15108802 Review.
Cited by
-
Role of Small GTPase RhoA in DNA Damage Response.Biomolecules. 2021 Feb 3;11(2):212. doi: 10.3390/biom11020212. Biomolecules. 2021. PMID: 33546351 Free PMC article. Review.
-
Rho GTPase independent regulation of ATM activation and cell survival by the RhoGEF Net1A.Cell Cycle. 2014;13(17):2765-72. doi: 10.4161/15384101.2015.945865. Cell Cycle. 2014. PMID: 25486363 Free PMC article.
-
Contributions of the RhoA guanine nucleotide exchange factor Net1 to polyoma middle T antigen-mediated mammary gland tumorigenesis and metastasis.Breast Cancer Res. 2018 May 16;20(1):41. doi: 10.1186/s13058-018-0966-2. Breast Cancer Res. 2018. PMID: 29769144 Free PMC article.
-
Interphase adhesion geometry is transmitted to an internal regulator for spindle orientation via caveolin-1.Nat Commun. 2016 Jun 13;7:ncomms11858. doi: 10.1038/ncomms11858. Nat Commun. 2016. PMID: 27292265 Free PMC article.
-
Neuroepithelial Cell Transforming Gene 1 Acts as an Oncogene and Is Mediated by miR-22 in Human Non-Small-Cell Lung Cancer.Biomed Res Int. 2020 Jan 11;2020:1648419. doi: 10.1155/2020/1648419. eCollection 2020. Biomed Res Int. 2020. PMID: 32420320 Free PMC article.
References
-
- Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–12161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

