An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody
- PMID: 23864620
- PMCID: PMC3754008
- DOI: 10.1128/JVI.00480-13
An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody
Abstract
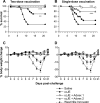
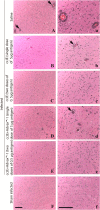
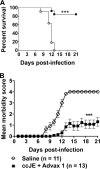
West Nile virus (WNV), currently the cause of a serious U.S. epidemic, is a mosquito-borne flavivirus and member of the Japanese encephalitis (JE) serocomplex. There is currently no approved human WNV vaccine, and treatment options remain limited, resulting in significant mortality and morbidity from human infection. Given the availability of approved human JE vaccines, this study asked whether the JE-ADVAX vaccine, which contains an inactivated cell culture JE virus antigen formulated with Advax delta inulin adjuvant, could provide heterologous protection against WNV infection in wild-type and β2-microglobulin-deficient (β2m(-/-)) murine models. Mice immunized twice or even once with JE-ADVAX were protected against lethal WNV challenge even when mice had low or absent serum cross-neutralizing WNV titers prior to challenge. Similarly, β2m(-/-) mice immunized with JE-ADVAX were protected against lethal WNV challenge in the absence of CD8(+) T cells and prechallenge WNV antibody titers. Protection against WNV could be adoptively transferred to naive mice by memory B cells from JE-ADVAX-immunized animals. Hence, in addition to increasing serum cross-neutralizing antibody titers, JE-ADVAX induced a memory B-cell population able to provide heterologous protection against WNV challenge. Heterologous protection was reduced when JE vaccine antigen was administered alone without Advax, confirming the importance of the adjuvant to induction of cross-protective immunity. In the absence of an approved human WNV vaccine, JE-ADVAX could provide an alternative approach for control of a major human WNV epidemic.
Figures

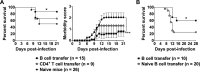
Similar articles
-
B cell response and mechanisms of antibody protection to West Nile virus.Viruses. 2014 Mar 3;6(3):1015-36. doi: 10.3390/v6031015. Viruses. 2014. PMID: 24594676 Free PMC article. Review.
-
Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection.Vaccine. 2015 Sep 11;33(38):4892-900. doi: 10.1016/j.vaccine.2015.07.051. Epub 2015 Jul 29. Vaccine. 2015. PMID: 26232344 Free PMC article.
-
An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses.J Gen Virol. 2010 Jun;91(Pt 6):1407-17. doi: 10.1099/vir.0.019190-0. Epub 2010 Feb 3. J Gen Virol. 2010. PMID: 20130134 Free PMC article.
-
JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody.J Virol. 2013 Apr;87(8):4395-402. doi: 10.1128/JVI.03144-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388724 Free PMC article.
-
Vaccines Against Dengue and West Nile Viruses in India: The Need of the Hour.Viral Immunol. 2020 Jul/Aug;33(6):423-433. doi: 10.1089/vim.2019.0122. Epub 2020 Apr 22. Viral Immunol. 2020. PMID: 32320353 Review.
Cited by
-
An Advax-Adjuvanted Inactivated Cell-Culture Derived Japanese Encephalitis Vaccine Induces Broadly Neutralising Anti-Flavivirus Antibodies, Robust Cellular Immunity and Provides Single Dose Protection.Vaccines (Basel). 2021 Oct 23;9(11):1235. doi: 10.3390/vaccines9111235. Vaccines (Basel). 2021. PMID: 34835166 Free PMC article.
-
B cell response and mechanisms of antibody protection to West Nile virus.Viruses. 2014 Mar 3;6(3):1015-36. doi: 10.3390/v6031015. Viruses. 2014. PMID: 24594676 Free PMC article. Review.
-
Advax4 delta inulin combination adjuvant together with ECMX, a fusion construct of four protective mTB antigens, induces a potent Th1 immune response and protects mice against Mycobacterium tuberculosis infection.Hum Vaccin Immunother. 2017 Dec 2;13(12):2967-2976. doi: 10.1080/21645515.2017.1368598. Hum Vaccin Immunother. 2017. PMID: 28937879 Free PMC article.
-
Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals.Vet Res. 2014 Dec 17;45(1):130. doi: 10.1186/s13567-014-0130-7. Vet Res. 2014. PMID: 25516480 Free PMC article.
-
Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection.Vaccine. 2015 Sep 11;33(38):4892-900. doi: 10.1016/j.vaccine.2015.07.051. Epub 2015 Jul 29. Vaccine. 2015. PMID: 26232344 Free PMC article.
References
-
- Cook S, Holmes EC. 2006. A multigene analysis of the phylogenetic relationships among the flaviviruses (family: Flaviviridae) and the evolution of vector transmission. Arch. Virol. 151:309–325 - PubMed
-
- Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 10:S98–S109 - PubMed
-
- Sips GJ, Wilschut J, Smit JM. 2012. Neuroinvasive flavivirus infections. Rev. Med. Virol. 22:69–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

