Three-dimensional structure of human NLRP10/PYNOD pyrin domain reveals a homotypic interaction site distinct from its mouse homologue
- PMID: 23861819
- PMCID: PMC3701624
- DOI: 10.1371/journal.pone.0067843
Three-dimensional structure of human NLRP10/PYNOD pyrin domain reveals a homotypic interaction site distinct from its mouse homologue
Abstract
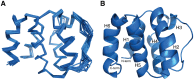
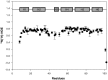
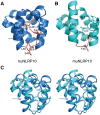
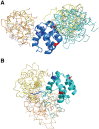
NLRPs (Nucleotide-binding domain, leucine-rich repeat and pyrin domain containing proteins) are a family of pattern-recognition receptors (PRRs) that sense intracellular microbial components and endogenous stress signals. NLRP10 (also known as PYNOD) is a unique NLRP member characterized by a lack of the putative ligand-binding leucine-rich repeat domain. Recently, human NLRP10 has been shown to inhibit the self-association of ASC into aggregates and ASC-mediated procaspase-1 processing. However, such activities are not found in mouse NLRP10. Here we report the solution structure and dynamics of human NLRP10 pyrin domain (PYD), whose helix H3 and loop H2-H3 adopt a conformation distinct from those of mouse NLRP10. Docking studies show that human and mouse NLRP10 PYDs may interact differently with ASC PYD. These results provide a possible structural explanation for the contrasting effect of NLRP10 on ASC aggregation in human cells versus mouse models. Finally, we also provide evidence that in human NLRP10 the PYD domain may not interact with the NOD domain to regulate its intrinsic nucleotide hydrolysis activity.
Conflict of interest statement
Figures

Similar articles
-
Mapping of POP1-binding site on pyrin domain of ASC.J Biol Chem. 2008 May 30;283(22):15390-8. doi: 10.1074/jbc.M801589200. Epub 2008 Mar 24. J Biol Chem. 2008. PMID: 18362139 Free PMC article.
-
Anti-inflammatory activity of PYNOD and its mechanism in humans and mice.J Immunol. 2010 May 15;184(10):5874-84. doi: 10.4049/jimmunol.0900779. Epub 2010 Apr 14. J Immunol. 2010. PMID: 20393137
-
Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly.J Biol Chem. 2013 May 10;288(19):13225-35. doi: 10.1074/jbc.M113.468033. Epub 2013 Mar 25. J Biol Chem. 2013. PMID: 23530044 Free PMC article.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Inhibiting the inflammasome: one domain at a time.Immunol Rev. 2015 May;265(1):205-16. doi: 10.1111/imr.12290. Immunol Rev. 2015. PMID: 25879295 Free PMC article. Review.
Cited by
-
Directionality of PYD filament growth determined by the transition of NLRP3 nucleation seeds to ASC elongation.Sci Adv. 2022 May 13;8(19):eabn7583. doi: 10.1126/sciadv.abn7583. Epub 2022 May 13. Sci Adv. 2022. PMID: 35559676 Free PMC article.
-
Crystal structure of the F27G AIM2 PYD mutant and similarities of its self-association to DED/DED interactions.J Mol Biol. 2014 Apr 3;426(7):1420-7. doi: 10.1016/j.jmb.2013.12.029. Epub 2014 Jan 7. J Mol Biol. 2014. PMID: 24406744 Free PMC article.
-
Membrane microdomains regulate NLRP10- and NLRP12-dependent signalling in A549 cells challenged with cigarette smoke extract.Arch Toxicol. 2018 May;92(5):1767-1783. doi: 10.1007/s00204-018-2185-0. Epub 2018 Apr 6. Arch Toxicol. 2018. PMID: 29623357 Free PMC article.
-
Activation and assembly of the inflammasomes through conserved protein domain families.Apoptosis. 2015 Feb;20(2):151-6. doi: 10.1007/s10495-014-1053-5. Apoptosis. 2015. PMID: 25398536 Free PMC article. Review.
-
NLRP3 inflammasome activation mechanism and its role in autoimmune liver disease.Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(11):1577-1586. doi: 10.3724/abbs.2022137. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36148948 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

