Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells
- PMID: 23846322
- PMCID: PMC3759676
- DOI: 10.18632/oncotarget.1070
Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells
Abstract
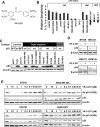
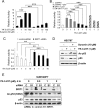
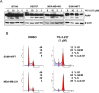
Many types of mutations in tumor suppressor p53 are oncogenic through gain-of-function. Therefore, targeting mutant p53 (mtp53) is a promising therapeutic approach to fight against many types of cancers. We report here a small molecule compound YK-3-237 that reduces acetylation of mtp53 and exhibits anti-proliferative effects toward triple-negative breast cancer (TNBC) cells carrying mtp53. YK-3-237 activates SIRT1 enzyme activities in vitro and deacetylation of both mtp53 in a SIRT1-dependent manner. Deacetylation of mtp53 resulted in depletion of mtp53 protein level and up-regulated the expression of WTp53-target genes, PUMA and NOXA. YK-3-237 also induces PARP-dependent apoptotic cell death and arrests the cell cycle at G2/M phase of mtp53 TNBC cells. Taken together, our data suggest that targeting acetylation of mtp53 is a potential target to treat human cancers.
Conflict of interest statement
Patent applications have been filed by Georgetown University on the behalf of the inventors that are listed as authors in this work.
Figures

Similar articles
-
Histone deacetylase inhibitors suppress mutant p53 transcription via HDAC8/YY1 signals in triple negative breast cancer cells.Cell Signal. 2016 May;28(5):506-515. doi: 10.1016/j.cellsig.2016.02.006. Epub 2016 Feb 11. Cell Signal. 2016. PMID: 26876786
-
Gain-of-Function Mutant p53 R273H Interacts with Replicating DNA and PARP1 in Breast Cancer.Cancer Res. 2020 Feb 1;80(3):394-405. doi: 10.1158/0008-5472.CAN-19-1036. Epub 2019 Nov 27. Cancer Res. 2020. PMID: 31776133 Free PMC article.
-
Targeting Triple Negative Breast Cancer with a Nucleus-Directed p53 Tetramerization Domain Peptide.Mol Pharm. 2021 Jan 4;18(1):338-346. doi: 10.1021/acs.molpharmaceut.0c00978. Epub 2020 Dec 8. Mol Pharm. 2021. PMID: 33289569 Free PMC article.
-
Role of Reactivating Mutant p53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer.Onco Targets Ther. 2022 Jan 8;15:23-30. doi: 10.2147/OTT.S342292. eCollection 2022. Onco Targets Ther. 2022. PMID: 35035222 Free PMC article. Review.
-
P53 mutations in triple negative breast cancer upregulate endosomal recycling of epidermal growth factor receptor (EGFR) increasing its oncogenic potency.Crit Rev Oncol Hematol. 2013 Nov;88(2):284-92. doi: 10.1016/j.critrevonc.2013.05.003. Epub 2013 Jun 5. Crit Rev Oncol Hematol. 2013. PMID: 23755891 Review.
Cited by
-
A Label-free Sirtuin 1 Assay based on Droplet-Electrospray Ionization Mass Spectrometry.Anal Methods. 2016 May 7;8(17):3458-3465. doi: 10.1039/C6AY00698A. Epub 2016 Apr 7. Anal Methods. 2016. PMID: 27482292 Free PMC article.
-
Dual SIRT1 expression patterns strongly suggests its bivalent role in human breast cancer.Oncotarget. 2017 Dec 6;8(67):110922-110930. doi: 10.18632/oncotarget.23006. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340027 Free PMC article.
-
Post-Translational Modifications (PTMs) of mutp53 and Epigenetic Changes Induced by mutp53.Biology (Basel). 2024 Jul 8;13(7):508. doi: 10.3390/biology13070508. Biology (Basel). 2024. PMID: 39056701 Free PMC article. Review.
-
Bromodomain Protein BRD4-Mediated Mutant p53 Transcription Promotes TNBC Progression.Int J Mol Sci. 2022 Dec 2;23(23):15163. doi: 10.3390/ijms232315163. Int J Mol Sci. 2022. PMID: 36499487 Free PMC article.
-
SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer.Oncotarget. 2018 Jul 17;9(55):30661-30678. doi: 10.18632/oncotarget.25771. eCollection 2018 Jul 17. Oncotarget. 2018. PMID: 30093977 Free PMC article.
References
-
- Criscitiello C, Azim HA, Jr, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Annals Oncol. 2012;23:vi13–18. - PubMed
-
- Crown J, O'Shaughnessy JO, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Annals Oncol. 2012;23:vi56–65. - PubMed
-
- Podo F, Buydens LMC, Degani H, Hihorst R, Klipp E, Gribbestad IS, Van Huffel S, van Laarhoven HW, Luts J, Monleon D, Postma GJ, Schneiderhan-Marra N, Santoro F, Wouters H, Russnes HG, Sørili T, et al. Triple-negative breast cancer: present challenges and new perspectives. Mol Oncol. 2010;4:209–229. - PMC - PubMed
-
- Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

