Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing
- PMID: 23844237
- PMCID: PMC3701065
- DOI: 10.1371/journal.pone.0068726
Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing
Abstract
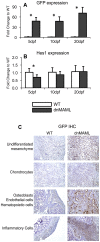
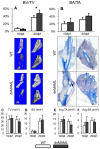
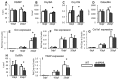
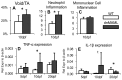
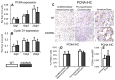
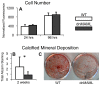
The Notch signaling pathway is an important regulator of embryological bone development, and many aspects of development are recapitulated during bone repair. We have previously reported that Notch signaling components are upregulated during bone fracture healing. However, the significance of the Notch pathway in bone regeneration has not been described. Therefore, the objective of this study was to determine the importance of Notch signaling in regulating bone fracture healing by using a temporally controlled inducible transgenic mouse model (Mx1-Cre;dnMAML(f/-)) to impair RBPjκ-mediated canonical Notch signaling. The Mx1 promoter was synthetically activated resulting in temporally regulated systemic dnMAML expression just prior to creation of bilateral tibial fractures. This allowed for mice to undergo unaltered embryological and post-natal skeletal development. Results showed that systemic Notch inhibition prolonged expression of inflammatory cytokines and neutrophil cell inflammation, and reduced the proportion of cartilage formation within the callus at 10 days-post-fracture (dpf) Notch inhibition did not affect early bone formation at 10dpf, but significantly altered bone maturation and remodeling at 20dpf. Increased bone volume fraction in dnMAML fractures, which was due to a moderate decrease in callus size with no change in bone mass, coincided with increased trabecular thickness but decreased connectivity density, indicating that patterning of bone was altered. Notch inhibition decreased total osteogenic cell density, which was comprised of more osteocytes rather than osteoblasts. dnMAML also decreased osteoclast density, suggesting that osteoclast activity may also be important for altered fracture healing. It is likely that systemic Notch inhibition had both direct effects within cell types as well as indirect effects initiated by temporally upstream events in the fracture healing cascade. Surprisingly, Notch inhibition did not alter cell proliferation. In conclusion, our results demonstrate that the Notch signaling pathway is required for the proper temporal progression of events required for successful bone fracture healing.
Conflict of interest statement
Figures

Similar articles
-
Fractures in geriatric mice show decreased callus expansion and bone volume.Clin Orthop Relat Res. 2014 Nov;472(11):3523-32. doi: 10.1007/s11999-014-3829-x. Epub 2014 Aug 9. Clin Orthop Relat Res. 2014. PMID: 25106797 Free PMC article.
-
Suppression of Notch Signaling in Osteoclasts Improves Bone Regeneration and Healing.J Orthop Res. 2019 Oct;37(10):2089-2103. doi: 10.1002/jor.24384. Epub 2019 Jun 24. J Orthop Res. 2019. PMID: 31166033 Free PMC article.
-
EP1(-/-) mice have enhanced osteoblast differentiation and accelerated fracture repair.J Bone Miner Res. 2011 Apr;26(4):792-802. doi: 10.1002/jbmr.272. J Bone Miner Res. 2011. PMID: 20939055 Free PMC article.
-
Role of bone regeneration and turnover modulators in control of fracture.Crit Rev Eukaryot Gene Expr. 2007;17(3):197-213. doi: 10.1615/critreveukargeneexpr.v17.i3.30. Crit Rev Eukaryot Gene Expr. 2007. PMID: 17725489 Review.
-
Expression of bone morphogenetic proteins in fracture healing.Clin Orthop Relat Res. 1998 Oct;(355 Suppl):S116-23. doi: 10.1097/00003086-199810001-00013. Clin Orthop Relat Res. 1998. PMID: 9917632 Review.
Cited by
-
Fractures in geriatric mice show decreased callus expansion and bone volume.Clin Orthop Relat Res. 2014 Nov;472(11):3523-32. doi: 10.1007/s11999-014-3829-x. Epub 2014 Aug 9. Clin Orthop Relat Res. 2014. PMID: 25106797 Free PMC article.
-
Inactivation of the Progesterone Receptor in Mx1+ Cells Potentiates Osteogenesis in Calvaria but Not in Long Bone.PLoS One. 2015 Oct 2;10(10):e0139490. doi: 10.1371/journal.pone.0139490. eCollection 2015. PLoS One. 2015. PMID: 26431032 Free PMC article.
-
Endothelial to mesenchymal Notch signaling regulates skeletal repair.JCI Insight. 2024 May 23;9(12):e181073. doi: 10.1172/jci.insight.181073. JCI Insight. 2024. PMID: 38781018 Free PMC article.
-
Effects of panax notoginseng saponins on the osteogenic differentiation of rabbit bone mesenchymal stem cells through TGF-β1 signaling pathway.BMC Complement Altern Med. 2016 Aug 26;16(1):319. doi: 10.1186/s12906-016-1304-9. BMC Complement Altern Med. 2016. PMID: 27561678 Free PMC article.
-
How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review.J Orthop Translat. 2019 Aug 8;21:111-121. doi: 10.1016/j.jot.2019.07.005. eCollection 2020 Mar. J Orthop Translat. 2019. PMID: 32309136 Free PMC article. Review.
References
-
- Braithwaite RS, Col NF, Wong JB (2003) Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc 51: 364-370. doi:10.1046/j.1532-5415.2003.51110.x. PubMed: 12588580. - DOI - PubMed
-
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 9: 66. doi:10.1186/1741-7015-9-66. PubMed: 21627784. - DOI - PMC - PubMed
-
- Carragee EJ, Hurwitz EL, Weiner BK, Bono CM, Rothman DJ (2011) Future directions for The spine journal: managing and reporting conflict of interest issues. Spine J 11: 695-697. doi:10.1016/j.spinee.2011.08.418. PubMed: 21925411. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

