Functional Role of mTORC2 versus Integrin-Linked Kinase in Mediating Ser473-Akt Phosphorylation in PTEN-Negative Prostate and Breast Cancer Cell Lines
- PMID: 23840605
- PMCID: PMC3686768
- DOI: 10.1371/journal.pone.0067149
Functional Role of mTORC2 versus Integrin-Linked Kinase in Mediating Ser473-Akt Phosphorylation in PTEN-Negative Prostate and Breast Cancer Cell Lines
Retraction in
-
Retraction: Functional Role of mTORC2 versus Integrin-Linked Kinase in Mediating Ser473-Akt Phosphorylation in PTEN-Negative Prostate and Breast Cancer Cell Lines.PLoS One. 2018 Aug 9;13(8):e0202299. doi: 10.1371/journal.pone.0202299. eCollection 2018. PLoS One. 2018. PMID: 30092069 Free PMC article. No abstract available.
Abstract
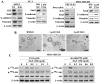
Although the rictor-mTOR complex (mTORC2) has been shown to act as phosphoinositide-dependent kinase (PDK)2 in many cell types, other kinases have also been implicated in mediating Ser473-Akt phosphorylation. Here, we demonstrated the cell line specificity of integrin-linked kinase (ILK) versus mTORC2 as PDK2 in LNCaP and PC-3 prostate and MDA-MB-468 breast cancer cells, of which the PTEN-negative status allowed the study of Ser473-Akt phosphorylation independent of external stimulation. PC-3 and MDA-MB-468 cells showed upregulated ILK expression relative to LNCaP cells, which expressed a high abundance of mTOR. Exposure to Ku-0063794, a second-generation mTOR inhibitor, decreased Ser473-Akt phosphorylation in LNCaP cells, but not in PC-3 or MDA-MB-468 cells. In contrast, treatment with T315, a novel ILK inhibitor, reduced the phosphorylation of Ser473-Akt in PC-3 and MDA-MB-468 cells without affecting that in LNCaP cells. This cell line specificity was verified by comparing Ser473-Akt phosphorylation status after genetic knockdown of rictor, ILK, and other putative Ser-473-Akt kinases. Genetic knockdown of rictor, but not ILK or the other kinases examined, inhibited Ser473-Akt phosphorylation in LNCaP cells. Conversely, PC-3 and MDA-MB-468 cells were susceptible to the effect of ILK silencing on Ser473-Akt phosphorylation, while knockdown of rictor or any of the other target kinases had no appreciable effect. Co-immunoprecipitation analysis demonstrated the physical interaction between ILK and Akt in PC-3 cells, and T315 blocked ILK-mediated Ser473 phosphorylation of bacterially expressed Akt. ILK also formed complexes with rictor in PC-3 and MDA-MB-468 cells that were disrupted by T315, but such complexes were not observed in LNCaP cells. In the PTEN-functional MDA-MB-231 cell line, both T315 and Ku-0063794 suppressed EGF-induced Ser473-Akt phosphorylation. Inhibition of ILK by T315 or siRNA-mediated knockdown suppressed epithelial-mesenchymal transition in MDA-MB-468 and PC-3 cells. Thus, we hypothesize that ILK might bestow growth advantage and metastatic potential in the course of tumor progression.
Conflict of interest statement
Figures






Similar articles
-
Receptor-specific mechanisms regulate phosphorylation of AKT at Ser473: role of RICTOR in β1 integrin-mediated cell survival.PLoS One. 2012;7(2):e32081. doi: 10.1371/journal.pone.0032081. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384145 Free PMC article.
-
Inhibition of 14-3-3 binding to Rictor of mTORC2 for Akt phosphorylation at Ser473 is regulated by selenoprotein W.Biochim Biophys Acta. 2013 Oct;1833(10):2135-42. doi: 10.1016/j.bbamcr.2013.05.005. Epub 2013 May 13. Biochim Biophys Acta. 2013. PMID: 23680186
-
Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival.Cancer Res. 2008 Mar 15;68(6):1618-24. doi: 10.1158/0008-5472.CAN-07-5869. Cancer Res. 2008. PMID: 18339839
-
Regulation of mTOR Signaling: Emerging Role of Cyclic Nucleotide-Dependent Protein Kinases and Implications for Cardiometabolic Disease.Int J Mol Sci. 2023 Jul 15;24(14):11497. doi: 10.3390/ijms241411497. Int J Mol Sci. 2023. PMID: 37511253 Free PMC article. Review.
-
Phosphorylation of Akt at the C-terminal tail triggers Akt activation.Cell Cycle. 2014;13(14):2162-4. doi: 10.4161/cc.29584. Epub 2014 Jun 16. Cell Cycle. 2014. PMID: 24933731 Free PMC article. Review.
Cited by
-
An In silico Approach to Identify High Affinity Small Molecule Targeting m-TOR Inhibitors for the Clinical Treatment of Breast Cancer.Asian Pac J Cancer Prev. 2019 Apr 29;20(4):1229-1241. doi: 10.31557/APJCP.2019.20.4.1229. Asian Pac J Cancer Prev. 2019. PMID: 31030499 Free PMC article.
-
A novel HIF-1α-integrin-linked kinase regulatory loop that facilitates hypoxia-induced HIF-1α expression and epithelial-mesenchymal transition in cancer cells.Oncotarget. 2015 Apr 10;6(10):8271-85. doi: 10.18632/oncotarget.3186. Oncotarget. 2015. PMID: 25821081 Free PMC article.
-
SPARC Controls Melanoma Cell Plasticity through Rac1.PLoS One. 2015 Aug 6;10(8):e0134714. doi: 10.1371/journal.pone.0134714. eCollection 2015. PLoS One. 2015. PMID: 26248315 Free PMC article.
-
Addressing the Reciprocal Crosstalk between the AR and the PI3K/AKT/mTOR Signaling Pathways for Prostate Cancer Treatment.Int J Mol Sci. 2023 Jan 24;24(3):2289. doi: 10.3390/ijms24032289. Int J Mol Sci. 2023. PMID: 36768610 Free PMC article. Review.
-
Oncogenic signaling in amphiregulin and EGFR-expressing PTEN-null human breast cancer.Mol Oncol. 2015 Feb;9(2):527-43. doi: 10.1016/j.molonc.2014.10.006. Epub 2014 Oct 23. Mol Oncol. 2015. PMID: 25454348 Free PMC article.
References
-
- Mora A, Komander D, van Aalten DM, Alessi DR (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170. - PubMed
-
- Dong LQ, Liu F (2005) PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab 289: E187–196. - PubMed
-
- Hresko RC, Mueckler M (2005) mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280: 40406–40416. - PubMed
-
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

