Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature
- PMID: 23839788
- PMCID: PMC3753390
- DOI: 10.1105/tpc.113.113118
Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature
Abstract
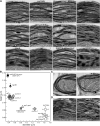
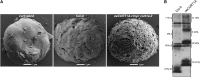
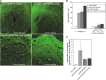
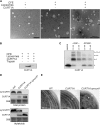
Chloroplasts of land plants characteristically contain grana, cylindrical stacks of thylakoid membranes. A granum consists of a core of appressed membranes, two stroma-exposed end membranes, and margins, which connect pairs of grana membranes at their lumenal sides. Multiple forces contribute to grana stacking, but it is not known how the extreme curvature at margins is generated and maintained. We report the identification of the CURVATURE THYLAKOID1 (CURT1) protein family, conserved in plants and cyanobacteria. The four Arabidopsis thaliana CURT1 proteins (CURT1A, B, C, and D) oligomerize and are highly enriched at grana margins. Grana architecture is correlated with the CURT1 protein level, ranging from flat lobe-like thylakoids with considerably fewer grana margins in plants without CURT1 proteins to an increased number of membrane layers (and margins) in grana at the expense of grana diameter in overexpressors of CURT1A. The endogenous CURT1 protein in the cyanobacterium Synechocystis sp PCC6803 can be partially replaced by its Arabidopsis counterpart, indicating that the function of CURT1 proteins is evolutionary conserved. In vitro, Arabidopsis CURT1A proteins oligomerize and induce tubulation of liposomes, implying that CURT1 proteins suffice to induce membrane curvature. We therefore propose that CURT1 proteins modify thylakoid architecture by inducing membrane curvature at grana margins.
Figures





Similar articles
-
Fine-Tuning of Photosynthesis Requires CURVATURE THYLAKOID1-Mediated Thylakoid Plasticity.Plant Physiol. 2018 Mar;176(3):2351-2364. doi: 10.1104/pp.17.00863. Epub 2018 Jan 26. Plant Physiol. 2018. PMID: 29374108 Free PMC article.
-
Curvature thylakoid 1 proteins modulate prolamellar body morphology and promote organized thylakoid biogenesis in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2021 Oct 19;118(42):e2113934118. doi: 10.1073/pnas.2113934118. Proc Natl Acad Sci U S A. 2021. PMID: 34654749 Free PMC article.
-
Thylakoid-Bound Polysomes and a Dynamin-Related Protein, FZL, Mediate Critical Stages of the Linear Chloroplast Biogenesis Program in Greening Arabidopsis Cotyledons.Plant Cell. 2018 Jul;30(7):1476-1495. doi: 10.1105/tpc.17.00972. Epub 2018 Jun 7. Plant Cell. 2018. PMID: 29880711 Free PMC article.
-
CURT1,CAAD-containing aaRSs, thylakoid curvature and gene translation.Trends Plant Sci. 2014 Feb;19(2):63-6. doi: 10.1016/j.tplants.2013.12.004. Epub 2013 Dec 30. Trends Plant Sci. 2014. PMID: 24388270 Review.
-
Structure and dynamics of thylakoids in land plants.J Exp Bot. 2014 May;65(8):1955-72. doi: 10.1093/jxb/eru090. Epub 2014 Mar 12. J Exp Bot. 2014. PMID: 24622954 Review.
Cited by
-
Comparative transcriptome analysis identified ChlH and POLGAMMA2 in regulating yellow-leaf coloration in Forsythia.Front Plant Sci. 2022 Sep 9;13:1009575. doi: 10.3389/fpls.2022.1009575. eCollection 2022. Front Plant Sci. 2022. PMID: 36160960 Free PMC article.
-
Thylakoid membrane stacking controls electron transport mode during the dark-to-light transition by adjusting the distances between PSI and PSII.Nat Plants. 2024 Mar;10(3):512-524. doi: 10.1038/s41477-024-01628-9. Epub 2024 Feb 23. Nat Plants. 2024. PMID: 38396112
-
Fundamental helical geometry consolidates the plant photosynthetic membrane.Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22366-22375. doi: 10.1073/pnas.1905994116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611387 Free PMC article.
-
The puzzle of chloroplast vesicle transport - involvement of GTPases.Front Plant Sci. 2014 Sep 23;5:472. doi: 10.3389/fpls.2014.00472. eCollection 2014. Front Plant Sci. 2014. PMID: 25295043 Free PMC article. Review.
-
The plant cytosolic m6A RNA methylome stabilizes photosynthesis in the cold.Plant Commun. 2023 Nov 13;4(6):100634. doi: 10.1016/j.xplc.2023.100634. Epub 2023 Jun 7. Plant Commun. 2023. PMID: 37287225 Free PMC article.
References
-
- Albertsson P.A., Andreasson E. (2004). The constant proportion of grana and stroma lamellae in plant chloroplasts. Physiol. Plant. 121: 334–342 - PubMed
-
- Allen J.F., Forsberg J. (2001). Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6: 317–326 - PubMed
-
- Anderson J.M. (1986). Photoregulation of the composition, function, and structure of thylakoid membranes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 37: 93–136
-
- Anderson J.M., Chow W.S., De Las Rivas J. (2008). Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynth. Res. 98: 575–587 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

