Genetic interaction between Kit and Scl
- PMID: 23836559
- PMCID: PMC3952531
- DOI: 10.1182/blood-2011-01-331819
Genetic interaction between Kit and Scl
Abstract
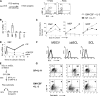
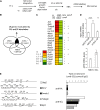
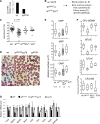
SCL/TAL1, a tissue-specific transcription factor of the basic helix-loop-helix family, and c-Kit, a tyrosine kinase receptor, control hematopoietic stem cell survival and quiescence. Here we report that SCL levels are limiting for the clonal expansion of Kit⁺ multipotent and erythroid progenitors. In addition, increased SCL expression specifically enhances the sensitivity of these progenitors to steel factor (KIT ligand) without affecting interleukin-3 response, whereas a DNA-binding mutant antagonizes KIT function and induces apoptosis in progenitors. Furthermore, a twofold increase in SCL levels in mice bearing a hypomorphic Kit allele (W41/41) corrects their hematocrits and deficiencies in erythroid progenitor numbers. At the molecular level, we found that SCL and c-Kit signaling control a common gene expression signature, of which 19 genes are associated with apoptosis. Half of those were decreased in purified megakaryocyte/erythroid progenitors (MEPs) from W41/41 mice and rescued by the SCL transgene. We conclude that Scl operates downstream of Kit to support the survival of MEPs. Finally, higher SCL expression upregulates Kit in normal bone marrow cells and increases chimerism after bone marrow transplantation, indicating that Scl is also upstream of Kit. We conclude that Scl and Kit establish a positive feedback loop in multipotent and MEPs.
Figures



Similar articles
-
The SCL complex regulates c-kit expression in hematopoietic cells through functional interaction with Sp1.Blood. 2002 Oct 1;100(7):2430-40. doi: 10.1182/blood-2002-02-0568. Blood. 2002. PMID: 12239153
-
Transcription factor SCL is required for c-kit expression and c-Kit function in hemopoietic cells.J Exp Med. 1998 Aug 3;188(3):439-50. doi: 10.1084/jem.188.3.439. J Exp Med. 1998. PMID: 9687522 Free PMC article.
-
Enhanced megakaryocyte and erythroid development from normal human CD34(+) cells: consequence of enforced expression of SCL.Blood. 1998 May 15;91(10):3756-65. Blood. 1998. PMID: 9573012
-
Kit and Scl regulation of hematopoietic stem cells.Curr Opin Hematol. 2014 Jul;21(4):256-64. doi: 10.1097/MOH.0000000000000052. Curr Opin Hematol. 2014. PMID: 24857885 Review.
-
SCL/TAL1 in Hematopoiesis and Cellular Reprogramming.Curr Top Dev Biol. 2016;118:163-204. doi: 10.1016/bs.ctdb.2016.01.004. Epub 2016 Feb 18. Curr Top Dev Biol. 2016. PMID: 27137657 Review.
Cited by
-
CD244 maintains the proliferation ability of leukemia initiating cells through SHP-2/p27kip1 signaling.Haematologica. 2017 Apr;102(4):707-718. doi: 10.3324/haematol.2016.151555. Epub 2017 Jan 25. Haematologica. 2017. PMID: 28126968 Free PMC article.
-
Hematopoietic stem and progenitor cell signaling in the niche.Leukemia. 2020 Dec;34(12):3136-3148. doi: 10.1038/s41375-020-01062-8. Epub 2020 Oct 19. Leukemia. 2020. PMID: 33077865 Review.
-
High-throughput screening in niche-based assay identifies compounds to target preleukemic stem cells.J Clin Invest. 2016 Dec 1;126(12):4569-4584. doi: 10.1172/JCI86489. Epub 2016 Oct 31. J Clin Invest. 2016. PMID: 27797342 Free PMC article.
-
GM-CSF Inhibits c-Kit and SCF Expression by Bone Marrow-Derived Dendritic Cells.Front Immunol. 2017 Feb 16;8:147. doi: 10.3389/fimmu.2017.00147. eCollection 2017. Front Immunol. 2017. PMID: 28261209 Free PMC article.
-
SCL, LMO1 and Notch1 reprogram thymocytes into self-renewing cells.PLoS Genet. 2014 Dec 18;10(12):e1004768. doi: 10.1371/journal.pgen.1004768. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25522233 Free PMC article.
References
-
- Laslo P, Spooner CJ, Warmflash A, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126(4):755–766. - PubMed
-
- Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14(7):1926–1930. - PubMed
-
- McCulloch EA, Siminovitch L, Till JE, Russell ES, Bernstein SE. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965;26(4):399–410. - PubMed
-
- McCulloch EA, Siminovitch L, Till JE. Spleen-colony formation in anemic mice of genotype WW. Science. 1964;144(3620):844–846. - PubMed
-
- Nocka K, Majumder S, Chabot B, et al. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989;3(6):816–826. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

