Impact of heat shock transcription factor 1 on global gene expression profiles in cells which induce either cytoprotective or pro-apoptotic response following hyperthermia
- PMID: 23834426
- PMCID: PMC3711851
- DOI: 10.1186/1471-2164-14-456
Impact of heat shock transcription factor 1 on global gene expression profiles in cells which induce either cytoprotective or pro-apoptotic response following hyperthermia
Abstract
Background: Elevated temperatures induce activation of the heat shock transcription factor 1 (HSF1) which in somatic cells leads to heat shock proteins synthesis and cytoprotection. However, in the male germ cells (spermatocytes) caspase-3 dependent apoptosis is induced upon HSF1 activation and spermatogenic cells are actively eliminated.
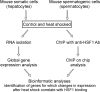
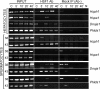
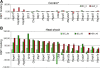
Results: To elucidate a mechanism of such diverse HSF1 activity we carried out genome-wide transcriptional analysis in control and heat-shocked cells, either spermatocytes or hepatocytes. Additionally, to identify direct molecular targets of active HSF1 we used chromatin immunoprecipitation assay (ChIP) combined with promoter microarrays (ChIP on chip). Genes that are differently regulated after HSF1 binding during hyperthermia in both types of cells have been identified. Despite HSF1 binding to promoter sequences in both types of cells, strong up-regulation of Hsps and other genes typically activated by the heat shock was observed only in hepatocytes. In spermatocytes HSF1 binding correlates with transcriptional repression on a large scale. HSF1-bound and negatively regulated genes encode mainly for proteins required for cell division, involved in RNA processing and piRNA biogenesis.
Conclusions: Observed suppression of the transcription could lead to genomic instability caused by meiotic recombination disturbances, which in turn might induce apoptosis of spermatogenic cells. We propose that HSF1-dependent induction of cell death is caused by the simultaneous repression of many genes required for spermatogenesis, which guarantees the elimination of cells damaged during heat shock. Such activity of HSF1 prevents transmission of damaged genetic material to the next generation.
Figures



Similar articles
-
Crosstalk between HSF1 and HSF2 during the heat shock response in mouse testes.Int J Biochem Cell Biol. 2014 Dec;57:76-83. doi: 10.1016/j.biocel.2014.10.006. Epub 2014 Oct 19. Int J Biochem Cell Biol. 2014. PMID: 25450459
-
Heat shock transcription factor 1 down-regulates spermatocyte-specific 70 kDa heat shock protein expression prior to the induction of apoptosis in mouse testes.Genes Cells. 2007 Apr;12(4):487-99. doi: 10.1111/j.1365-2443.2007.01069.x. Genes Cells. 2007. PMID: 17397396
-
Spermatocyte-specific expression of constitutively active heat shock factor 1 induces HSP70i-resistant apoptosis in male germ cells.Cell Death Differ. 2006 Feb;13(2):212-22. doi: 10.1038/sj.cdd.4401758. Cell Death Differ. 2006. PMID: 16151457
-
The Role of Heat Shock Factors in Mammalian Spermatogenesis.Adv Anat Embryol Cell Biol. 2017;222:45-65. doi: 10.1007/978-3-319-51409-3_3. Adv Anat Embryol Cell Biol. 2017. PMID: 28389750 Review.
-
[The heat shock response and HSP70 gene expression in male germ cells].Postepy Biochem. 2006;52(3):289-95. Postepy Biochem. 2006. PMID: 17201064 Review. Polish.
Cited by
-
PHLDA1 Does Not Contribute Directly to Heat Shock-Induced Apoptosis of Spermatocytes.Int J Mol Sci. 2019 Dec 30;21(1):267. doi: 10.3390/ijms21010267. Int J Mol Sci. 2019. PMID: 31906015 Free PMC article.
-
First Insights on the Presence of the Unfolded Protein Response in Human Spermatozoa.Int J Mol Sci. 2019 Nov 5;20(21):5518. doi: 10.3390/ijms20215518. Int J Mol Sci. 2019. PMID: 31694346 Free PMC article.
-
A combined approach to heat stress effect on male fertility in Nasonia vitripennis: from the physiological consequences on spermatogenesis to the reproductive adjustment of females mated with stressed males.PLoS One. 2015 Mar 25;10(3):e0120656. doi: 10.1371/journal.pone.0120656. eCollection 2015. PLoS One. 2015. PMID: 25807005 Free PMC article.
-
Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches.Antioxidants (Basel). 2022 Mar 24;11(4):625. doi: 10.3390/antiox11040625. Antioxidants (Basel). 2022. PMID: 35453310 Free PMC article. Review.
-
Identification of Key Genes Affecting Results of Hyperthermia in Osteosarcoma Based on Integrative ChIP-Seq/TargetScan Analysis.Med Sci Monit. 2017 Apr 28;23:2042-2048. doi: 10.12659/msm.901191. Med Sci Monit. 2017. PMID: 28453502 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

