Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis
- PMID: 23834359
- PMCID: PMC3710246
- DOI: 10.1186/1471-2121-14-32
Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis
Abstract
Background: Apoptosis is a form of programmed cell death that is regulated by the Bcl-2 family and caspase family of proteins. The caspase cascade responsible for executing cell death following cytochrome c release is well described; however the distinct roles of caspases-9, -3 and -7 during this process are not completely defined.
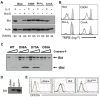
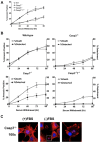
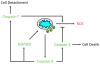
Results: Here we demonstrate several unique functions for each of these caspases during cell death. Specific inhibition of caspase-9 allows for efficient release of cytochrome c, but blocks changes in mitochondrial morphology and ROS production. We show that caspase-9 can cleave Bid into tBid at amino acid 59 and that this cleavage of Bid is required for ROS production following serum withdrawal. We also demonstrate that caspase-3-deficient MEFs are less sensitive to intrinsic cell death stimulation, yet have higher ROS production. In contrast, caspase-7-deficient MEFs are not resistance to intrinsic cell death, but remain attached to the ECM.
Conclusions: Taken together, these data suggest that caspase-9 is required for mitochondrial morphological changes and ROS production by cleaving and activating Bid into tBid. After activation by caspase-9, caspase-3 inhibits ROS production and is required for efficient execution of apoptosis, while effector caspase-7 is required for apoptotic cell detachment.
Figures


Similar articles
-
The caspase-8/Bid/cytochrome c axis links signals from death receptors to mitochondrial reactive oxygen species production.Free Radic Biol Med. 2017 Nov;112:567-577. doi: 10.1016/j.freeradbiomed.2017.09.001. Epub 2017 Sep 6. Free Radic Biol Med. 2017. PMID: 28888620
-
Caspase-9 and effector caspases have sequential and distinct effects on mitochondria.Oncogene. 2005 Sep 22;24(42):6354-66. doi: 10.1038/sj.onc.1208793. Oncogene. 2005. PMID: 16007191
-
Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis.J Biol Chem. 2007 May 11;282(19):14132-9. doi: 10.1074/jbc.M700077200. Epub 2007 Mar 19. J Biol Chem. 2007. PMID: 17371867
-
What are caspases 3 and 7 doing upstream of the mitochondria?Cancer Biol Ther. 2006 Jul;5(7):763-5. doi: 10.4161/cbt.5.7.3228. Epub 2006 Jul 26. Cancer Biol Ther. 2006. PMID: 16921264 Review.
-
Cytochrome c: the Achilles' heel in apoptosis.Cell Mol Life Sci. 2012 Jun;69(11):1787-97. doi: 10.1007/s00018-011-0895-z. Epub 2011 Dec 17. Cell Mol Life Sci. 2012. PMID: 22179840 Free PMC article. Review.
Cited by
-
A Combined Antitumor Strategy Mediated by a New Targeted Nanosystem to Hepatocellular Carcinoma.Int J Nanomedicine. 2021 May 18;16:3385-3405. doi: 10.2147/IJN.S302288. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34040370 Free PMC article.
-
Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells.PLoS One. 2021 Mar 16;16(3):e0248521. doi: 10.1371/journal.pone.0248521. eCollection 2021. PLoS One. 2021. PMID: 33725002 Free PMC article.
-
MFG-E8 Exerts Neuroprotection in Neural Stem Cells Induced by Anesthetic Sevoflurane via Regulating the PI3K/AKT Pathways.Stem Cells Int. 2022 Oct 12;2022:5609501. doi: 10.1155/2022/5609501. eCollection 2022. Stem Cells Int. 2022. Retraction in: Stem Cells Int. 2024 Jan 24;2024:9836351. doi: 10.1155/2024/9836351. PMID: 36277041 Free PMC article. Retracted.
-
Noncanonical Cell Death Induction by Reassortant Reovirus.J Virol. 2020 Oct 27;94(22):e01613-20. doi: 10.1128/JVI.01613-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847857 Free PMC article.
-
Ponicidin Promotes Hepatocellular Carcinoma Mitochondrial Apoptosis by Stabilizing Keap1-PGAM5 Complex.Adv Sci (Weinh). 2024 Oct;11(38):e2406080. doi: 10.1002/advs.202406080. Epub 2024 Aug 8. Adv Sci (Weinh). 2024. PMID: 39116422 Free PMC article.
References
-
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S. et al.Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

