Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction
- PMID: 23831726
- PMCID: PMC3736338
- DOI: 10.1038/ncb2796
Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction
Abstract
Apical constriction promotes epithelia folding, which changes tissue architecture. During Drosophila gastrulation, mesoderm cells exhibit repeated contractile pulses that are stabilized such that cells apically constrict like a ratchet. The transcription factor Twist is required to stabilize cell shape. However, it is unknown how Twist spatially coordinates downstream signals to prevent cell relaxation. We find that during constriction, Rho-associated kinase (Rok) is polarized to the middle of the apical domain (medioapical cortex), separate from adherens junctions. Rok recruits or stabilizes medioapical myosin II (Myo-II), which contracts dynamic medioapical actin cables. The formin Diaphanous mediates apical actin assembly to suppress medioapical E-cadherin localization and form stable connections between the medioapical contractile network and adherens junctions. Twist is not required for apical Rok recruitment, but instead polarizes Rok medioapically. Therefore, Twist establishes radial cell polarity of Rok/Myo-II and E-cadherin and promotes medioapical actin assembly in mesoderm cells to stabilize cell shape fluctuations.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

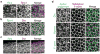
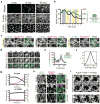
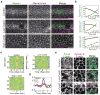
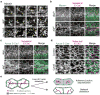
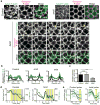

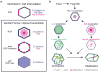
Comment in
-
Gastrulation: cell polarity comes full circle.Curr Biol. 2013 Sep 23;23(18):R845-8. doi: 10.1016/j.cub.2013.08.003. Curr Biol. 2013. PMID: 24070446
Similar articles
-
folded gastrulation, cell shape change and the control of myosin localization.Development. 2005 Sep;132(18):4165-78. doi: 10.1242/dev.01938. Development. 2005. PMID: 16123312
-
Pulsed contractions of an actin-myosin network drive apical constriction.Nature. 2009 Jan 22;457(7228):495-9. doi: 10.1038/nature07522. Epub 2008 Nov 23. Nature. 2009. PMID: 19029882 Free PMC article.
-
Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis.J Cell Biol. 2014 Aug 4;206(3):435-50. doi: 10.1083/jcb.201402004. J Cell Biol. 2014. PMID: 25092658 Free PMC article.
-
Compartmentalized morphogenesis in epithelia: from cell to tissue shape.Dev Dyn. 2005 Mar;232(3):685-94. doi: 10.1002/dvdy.20334. Dev Dyn. 2005. PMID: 15712202 Review.
-
The Fog signaling pathway: insights into signaling in morphogenesis.Dev Biol. 2014 Oct 1;394(1):6-14. doi: 10.1016/j.ydbio.2014.08.003. Epub 2014 Aug 12. Dev Biol. 2014. PMID: 25127992 Free PMC article. Review.
Cited by
-
β-H-Spectrin is a key component of an apical-medial hub of proteins during cell wedging in tube morphogenesis.J Cell Sci. 2024 Aug 1;137(15):jcs261946. doi: 10.1242/jcs.261946. Epub 2024 Aug 12. J Cell Sci. 2024. PMID: 38988298 Free PMC article.
-
Apical Sarcomere-like Actomyosin Contracts Nonmuscle Drosophila Epithelial Cells.Dev Cell. 2016 Nov 7;39(3):346-358. doi: 10.1016/j.devcel.2016.09.023. Epub 2016 Oct 20. Dev Cell. 2016. PMID: 27773487 Free PMC article.
-
A coarse-grained approach to model the dynamics of the actomyosin cortex.BMC Biol. 2022 Apr 22;20(1):90. doi: 10.1186/s12915-022-01279-2. BMC Biol. 2022. PMID: 35459165 Free PMC article.
-
The Drosophila anterior-posterior axis is polarized by asymmetric myosin activation.Curr Biol. 2022 Jan 24;32(2):374-385.e4. doi: 10.1016/j.cub.2021.11.024. Epub 2021 Dec 1. Curr Biol. 2022. PMID: 34856125 Free PMC article.
-
Wounded cells drive rapid epidermal repair in the early Drosophila embryo.Mol Biol Cell. 2013 Oct;24(20):3227-37. doi: 10.1091/mbc.E13-05-0228. Epub 2013 Aug 28. Mol Biol Cell. 2013. PMID: 23985320 Free PMC article.
References
-
- Leptin M. Gastrulation movements: the logic and the nuts and bolts. Dev Cell. 2005;8:305–320. - PubMed
-
- Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–462. - PubMed
-
- Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

