Brain tumor stem cells: Molecular characteristics and their impact on therapy
- PMID: 23831316
- PMCID: PMC3866208
- DOI: 10.1016/j.mam.2013.06.004
Brain tumor stem cells: Molecular characteristics and their impact on therapy
Abstract
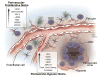
Glioblastoma (GBM) is the most prevalent primary brain tumor and ranks among the most lethal of human cancers with conventional therapy offering only palliation. Great strides have been made in understanding brain cancer genetics and modeling these tumors with new targeted therapies being tested, but these advances have not translated into substantially improved patient outcomes. Multiple chemotherapeutic agents, including temozolomide, the first-line treatment for glioblastoma, have been developed to kill cancer cells. However, the response to temozolomide in GBM is modest. Radiation is also moderately effective but this approach is plagued by limitations due to collateral radiation damage to healthy brain tissue and development of radioresistance. Therapeutic resistance is attributed at least in part to a cell population within the tumor that possesses stem-like characteristics and tumor propagating capabilities, referred to as cancer stem cells. Within GBM, the intratumoral heterogeneity is derived from a combination of regional genetic variance and a cellular hierarchy often regulated by distinct cancer stem cell niches, most notably perivascular and hypoxic regions. With the recent emergence as a key player in tumor biology, cancer stem cells have symbiotic relationships with the tumor microenvironment, oncogenic signaling pathways, and epigenetic modifications. The origins of cancer stem cells and their contributions to brain tumor growth and therapeutic resistance are under active investigation with novel anti-cancer stem cell therapies offering potential new hope for this lethal disease.
Keywords: Epigenetics; Glioma stem cells; Hypoxia; Microenvironment; Therapeutic resistance.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):346-354. doi: 10.1016/j.bbcan.2018.04.008. Epub 2018 Apr 21. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29684521 Review.
-
Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma.Cells. 2021 Mar 11;10(3):621. doi: 10.3390/cells10030621. Cells. 2021. PMID: 33799798 Free PMC article. Review.
-
Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting.Protein Cell. 2010 Jul;1(7):638-55. doi: 10.1007/s13238-010-0078-y. Epub 2010 Jul 29. Protein Cell. 2010. PMID: 21203936 Free PMC article. Review.
-
LGR5 promotes tumorigenicity and invasion of glioblastoma stem-like cells and is a potential therapeutic target for a subset of glioblastoma patients.J Pathol. 2019 Feb;247(2):228-240. doi: 10.1002/path.5186. Epub 2019 Jan 10. J Pathol. 2019. PMID: 30357839
-
The Importance of Tumor Stem Cells in Glioblastoma Resistance to Therapy.Int J Mol Sci. 2021 Apr 8;22(8):3863. doi: 10.3390/ijms22083863. Int J Mol Sci. 2021. PMID: 33917954 Free PMC article. Review.
Cited by
-
An Inflammatory Response-Related Gene Signature Can Impact the Immune Status and Predict the Prognosis of Hepatocellular Carcinoma.Front Oncol. 2021 Mar 22;11:644416. doi: 10.3389/fonc.2021.644416. eCollection 2021. Front Oncol. 2021. PMID: 33828988 Free PMC article.
-
Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma.Drug Des Devel Ther. 2015 Apr 9;9:2089-100. doi: 10.2147/DDDT.S79592. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25926719 Free PMC article. Review.
-
Growth factor independence underpins a paroxysmal, aggressive Wnt5aHigh/EphA2Low phenotype in glioblastoma stem cells, conducive to experimental combinatorial therapy.J Exp Clin Cancer Res. 2022 Apr 12;41(1):139. doi: 10.1186/s13046-022-02333-1. J Exp Clin Cancer Res. 2022. PMID: 35414102 Free PMC article.
-
Significance of Glioma Stem-Like Cells in the Tumor Periphery That Express High Levels of CD44 in Tumor Invasion, Early Progression, and Poor Prognosis in Glioblastoma.Stem Cells Int. 2018 Aug 23;2018:5387041. doi: 10.1155/2018/5387041. eCollection 2018. Stem Cells Int. 2018. PMID: 30210550 Free PMC article.
-
Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy.Cancers (Basel). 2022 Jul 31;14(15):3743. doi: 10.3390/cancers14153743. Cancers (Basel). 2022. PMID: 35954407 Free PMC article. Review.
References
-
- Acquati S, Greco A, Licastro D, Bhagat H, Ceric D, Rossini Z, Grieve J, Shaked-Rabi M, Henriquez NV, Brandner S, Stupka E, Marino S. Epigenetic regulation of survivin by Bmi1 is cell type specific during corticogenesis and in gliomas. Stem Cells Dayt Ohio. 2013;31:190–202. - PubMed
-
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

