A quantitative infection assay for human type I, II, and III interferon antiviral activities
- PMID: 23829314
- PMCID: PMC3716869
- DOI: 10.1186/1743-422X-10-224
A quantitative infection assay for human type I, II, and III interferon antiviral activities
Abstract
Background: Upon virus infection, cells secrete a diverse group of antiviral molecules that signal proximal cells to enter into an antiviral state, slowing or preventing viral spread. These paracrine signaling molecules can work synergistically, so measurement of any one antiviral molecule does not reflect the total antiviral activity of the system.
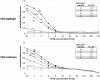
Results: We have developed an antiviral assay based on replication inhibition of an engineered fluorescent vesicular stomatitis virus reporter strain on A549 human lung epithelial cells. Our assay provides a quantitative functional readout of human type I, II, and III interferon activities, and it provides better sensitivity, intra-, and inter-assay reproducibility than the traditional crystal violet based assay. Further, it eliminates cell fixation, rinsing, and staining steps, and is inexpensive to implement.
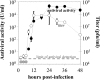
Conclusions: A dsRed2-strain of vesicular stomatitis virus that is sensitive to type I, II, and III interferons was used to develop a convenient and sensitive assay for interferon antiviral activity. We demonstrate use of the assay to quantify the kinetics of paracrine antiviral signaling from human prostate cancer (PC3) cells in response to viral infection. The assay is applicable to high-throughput screening for anti-viral compounds as well as basic studies of cellular antiviral signaling.
Figures



Similar articles
-
A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon.PLoS One. 2011;6(10):e25858. doi: 10.1371/journal.pone.0025858. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998709 Free PMC article.
-
Establishing a safe, rapid, convenient and low-cost antiviral assay of interferon bioactivity based on recombinant VSV expressing GFP.J Virol Methods. 2018 Feb;252:1-7. doi: 10.1016/j.jviromet.2017.08.007. Epub 2017 Aug 20. J Virol Methods. 2018. PMID: 28834736
-
[Use of Getah virus for antiviral assay of human interferon].Uirusu. 2005 Dec;55(2):317-26. doi: 10.2222/jsv.55.317. Uirusu. 2005. PMID: 16557019 Japanese.
-
Biological assays for interferons.J Immunol Methods. 2002 Mar 1;261(1-2):21-36. doi: 10.1016/s0022-1759(01)00570-1. J Immunol Methods. 2002. PMID: 11861063 Review.
-
[The present state of research in direct antiviral mechanism of interferon on hepatitis B virus].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Dec;26(6):1358-62, 1371. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 20095503 Review. Chinese.
Cited by
-
The mRNA Cap 2'-O-Methyltransferase CMTR1 Regulates the Expression of Certain Interferon-Stimulated Genes.mSphere. 2020 May 13;5(3):e00202-20. doi: 10.1128/mSphere.00202-20. mSphere. 2020. PMID: 32404510 Free PMC article.
-
Genome rearrangement affects RNA virus adaptability on prostate cancer cells.Front Genet. 2015 Apr 1;6:121. doi: 10.3389/fgene.2015.00121. eCollection 2015. Front Genet. 2015. PMID: 25883601 Free PMC article.
-
Illumina MiSeq sequencing disfavours a sequence motif in the GFP reporter gene.Sci Rep. 2016 May 19;6:26314. doi: 10.1038/srep26314. Sci Rep. 2016. PMID: 27193250 Free PMC article.
-
Kinetic Differences and Synergistic Antiviral Effects Between Type I and Type III Interferon Signaling Indicate Pathway Independence.J Interferon Cytokine Res. 2015 Sep;35(9):734-47. doi: 10.1089/jir.2015.0008. Epub 2015 May 4. J Interferon Cytokine Res. 2015. PMID: 25938799 Free PMC article.
-
Recombinant IFN-γ from the bank vole Myodes glareolus: a novel tool for research on rodent reservoirs of zoonotic pathogens.Sci Rep. 2018 Feb 12;8(1):2797. doi: 10.1038/s41598-018-21143-0. Sci Rep. 2018. PMID: 29434310 Free PMC article.
References
-
- Kawaguchi S, Ishiguro Y, Imaizumi T, Mori F, Matsumiya T, Yoshida H, Ota K, Sakuraba H, Yamagata K, Sato Y. et al.Retinoic acid-inducible gene-I is constitutively expressed and involved in IFN-gamma-stimulated CXCL9-11 production in intestinal epithelial cells. Immunol Lett. 2009;10:9–13. doi: 10.1016/j.imlet.2009.01.008. - DOI - PubMed
-
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;10:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

