P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo
- PMID: 23828651
- PMCID: PMC3889391
- DOI: 10.1007/s11302-013-9374-3
P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo
Abstract
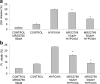
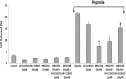
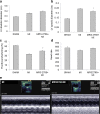
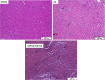
Extracellular nucleotides acting via P2 receptors play important roles in cardiovascular physiology/pathophysiology. Pyrimidine nucleotides activate four G protein-coupled P2Y receptors (P2YRs): P2Y2 and P2Y4 (UTP-activated), P2Y6, and P2Y14. Previously, we showed that uridine 5'-triphosphate (UTP) activating P2Y2R reduced infarct size and improved mouse heart function after myocardial infarct (MI). Here, we examined the cardioprotective role of P2Y2R in vitro and in vivo following MI using uridine-5'-tetraphosphate δ-phenyl ester tetrasodium salt (MRS2768), a selective and more stable P2Y2R agonist. Cultured rat cardiomyocytes pretreated with MRS2768 displayed protection from hypoxia [as revealed by lactate dehydrogenase (LDH) release and propidium iodide (PI) binding], which was reduced by P2Y2R antagonist, AR-C118925 (5-((5-(2,8-dimethyl-5H-dibenzo[a,d][7]annulen-5-yl)-2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)-N-(1H-tetrazol-5-yl)furan-2-carboxamide). In vivo, echocardiography and infarct size staining of triphenyltetrazolium chloride (TTC) in 3 groups of mice 24 h post-MI: sham, MI, and MI+MRS2768 indicated protection. Fractional shortening (FS) was higher in MRS2768-treated mice than in MI alone (40.0 ± 3.1 % vs. 33.4 ± 2.7 %, p < 0.001). Troponin T and tumor necrosis factor-α (TNF-α) measurements demonstrated that MRS2768 pretreatment reduced myocardial damage (p < 0.05) and c-Jun phosphorylation increased. Thus, P2Y2R activation protects cardiomyocytes from hypoxia in vitro and reduces post-ischemic myocardial damage in vivo.
Figures


Similar articles
-
UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor.Biochem Pharmacol. 2011 Nov 1;82(9):1126-33. doi: 10.1016/j.bcp.2011.07.094. Epub 2011 Aug 3. Biochem Pharmacol. 2011. PMID: 21839729
-
Uridine-5'-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct.Biochem Pharmacol. 2006 Oct 16;72(8):949-55. doi: 10.1016/j.bcp.2006.07.019. Epub 2006 Aug 30. Biochem Pharmacol. 2006. PMID: 16939682 Free PMC article.
-
P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling.Circ Res. 2017 Nov 10;121(11):1224-1236. doi: 10.1161/CIRCRESAHA.117.310812. Epub 2017 Sep 18. Circ Res. 2017. PMID: 28923792 Free PMC article.
-
Tools and drugs for uracil nucleotide-activated P2Y receptors.Pharmacol Ther. 2018 Oct;190:24-80. doi: 10.1016/j.pharmthera.2018.04.002. Epub 2018 Apr 13. Pharmacol Ther. 2018. PMID: 29660366 Review.
-
P2 receptors activated by uracil nucleotides--an update.Curr Med Chem. 2006;13(3):289-312. doi: 10.2174/092986706775476052. Curr Med Chem. 2006. PMID: 16475938 Review.
Cited by
-
Extracellular nucleotide signaling in solid organ transplantation.Am J Transplant. 2020 Mar;20(3):633-640. doi: 10.1111/ajt.15651. Epub 2019 Nov 4. Am J Transplant. 2020. PMID: 31605463 Free PMC article. Review.
-
P2Y2 receptor decreases blood pressure by inhibiting ENaC.JCI Insight. 2023 Jul 24;8(14):e167704. doi: 10.1172/jci.insight.167704. JCI Insight. 2023. PMID: 37279066 Free PMC article.
-
Cardiac purinergic signalling in health and disease.Purinergic Signal. 2015 Mar;11(1):1-46. doi: 10.1007/s11302-014-9436-1. Epub 2014 Dec 20. Purinergic Signal. 2015. PMID: 25527177 Free PMC article. Review.
-
Characterisation of P2Y2 receptors in human vascular endothelial cells using AR-C118925XX, a competitive and selective P2Y2 antagonist.Br J Pharmacol. 2019 Aug;176(16):2894-2904. doi: 10.1111/bph.14715. Epub 2019 Jul 6. Br J Pharmacol. 2019. PMID: 31116875 Free PMC article.
-
Metabolite G-Protein Coupled Receptors in Cardio-Metabolic Diseases.Cells. 2021 Nov 29;10(12):3347. doi: 10.3390/cells10123347. Cells. 2021. PMID: 34943862 Free PMC article. Review.
References
-
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

