Estrogen receptor α can selectively repress dioxin receptor-mediated gene expression by targeting DNA methylation
- PMID: 23828038
- PMCID: PMC3783176
- DOI: 10.1093/nar/gkt595
Estrogen receptor α can selectively repress dioxin receptor-mediated gene expression by targeting DNA methylation
Abstract
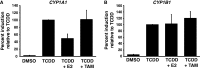
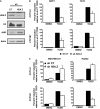
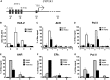
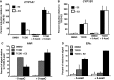
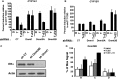
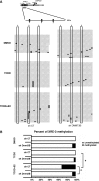
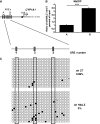
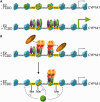
Selective inhibitory crosstalk has been known to occur within the signaling pathways of the dioxin (AhR) and estrogen (ERα) receptors. More specifically, ERα represses a cytochrome P450-encoding gene (CYP1A1) that converts cellular estradiol into a metabolite that inhibits the cell cycle, while it has no effect on a P450-encoding gene (CYP1B1) that converts estrodiol into a genotoxic product. Here we show that ERα represses CYP1A1 by targeting the Dnmt3B DNA methyltransferase and concomitant DNA methylation of the promoter. We also find that histone H2A.Z can positively contribute to CYP1A1 gene expression, and its presence at that gene is inversely correlated with DNA methylation. Taken together, our results provide a framework for how ERα can repress transcription, and how that impinges on the production of an enzyme that generates genotoxic estradiol metabolites, and potential breast cancer progression. Finally, our results reveal a new mechanism for how H2A.Z can positively influence gene expression, which is by potentially competing with DNA methylation events in breast cancer cells.
Figures
Similar articles
-
Dioxin and estrogen signaling in lung adenocarcinoma cells with different aryl hydrocarbon receptor/estrogen receptor α phenotypes.Am J Respir Cell Mol Biol. 2013 Dec;49(6):1064-73. doi: 10.1165/rcmb.2012-0497OC. Am J Respir Cell Mol Biol. 2013. PMID: 23855798 Free PMC article.
-
12-O-tetradecanoylphorbol-13-acetate upregulates the Ah receptor and differentially alters CYP1B1 and CYP1A1 expression in MCF-7 breast cancer cells.J Cell Biochem. 1998 Sep 1;70(3):289-96. J Cell Biochem. 1998. PMID: 9706865
-
Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells.Cancer Lett. 2010 Dec 28;299(2):119-29. doi: 10.1016/j.canlet.2010.08.010. Epub 2010 Sep 16. Cancer Lett. 2010. PMID: 20846786 Free PMC article.
-
Defining molecular sensors to assess long-term effects of pesticides on carcinogenesis.Int J Mol Sci. 2014 Sep 25;15(9):17148-61. doi: 10.3390/ijms150917148. Int J Mol Sci. 2014. PMID: 25257533 Free PMC article. Review.
-
Induction of drug-metabolizing enzymes by dioxin.Drug Metab Rev. 1997 Nov;29(4):1107-27. doi: 10.3109/03602539709002245. Drug Metab Rev. 1997. PMID: 9421687 Review. No abstract available.
Cited by
-
The Multiple Biological Targets of Hops and Bioactive Compounds.Chem Res Toxicol. 2019 Feb 18;32(2):222-233. doi: 10.1021/acs.chemrestox.8b00345. Epub 2019 Jan 22. Chem Res Toxicol. 2019. PMID: 30608650 Free PMC article. Review.
-
Estradiol-Induced Epigenetically Mediated Mechanisms and Regulation of Gene Expression.Int J Mol Sci. 2020 Apr 30;21(9):3177. doi: 10.3390/ijms21093177. Int J Mol Sci. 2020. PMID: 32365920 Free PMC article. Review.
-
Berberine Activates Aryl Hydrocarbon Receptor but Suppresses CYP1A1 Induction through miR-21-3p Stimulation in MCF-7 Breast Cancer Cells.Molecules. 2017 Oct 28;22(11):1847. doi: 10.3390/molecules22111847. Molecules. 2017. PMID: 29143794 Free PMC article.
-
Genes, Gender, Environment, and Novel Functions of Estrogen Receptor Beta in the Susceptibility to Neurodevelopmental Disorders.Brain Sci. 2017 Feb 23;7(3):24. doi: 10.3390/brainsci7030024. Brain Sci. 2017. PMID: 28241485 Free PMC article. Review.
-
Red Clover Aryl Hydrocarbon Receptor (AhR) and Estrogen Receptor (ER) Agonists Enhance Genotoxic Estrogen Metabolism.Chem Res Toxicol. 2017 Nov 20;30(11):2084-2092. doi: 10.1021/acs.chemrestox.7b00237. Epub 2017 Oct 19. Chem Res Toxicol. 2017. PMID: 28985473 Free PMC article.
References
-
- Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47:6517–6521. - PubMed
-
- Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. - PubMed
-
- Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. - PubMed
-
- Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol. Ther. 1993;57:237–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

