Cannabinoid Receptor 2 (CB2) Plays a Role in the Generation of Germinal Center and Memory B Cells, but Not in the Production of Antigen-Specific IgG and IgM, in Response to T-dependent Antigens
- PMID: 23826323
- PMCID: PMC3695093
- DOI: 10.1371/journal.pone.0067587
Cannabinoid Receptor 2 (CB2) Plays a Role in the Generation of Germinal Center and Memory B Cells, but Not in the Production of Antigen-Specific IgG and IgM, in Response to T-dependent Antigens
Abstract
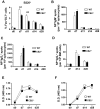
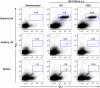
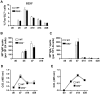
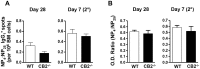
The cannabinoid receptor 2 (CB2) has been reported to modulate B cell functions including migration, proliferation and isotype class switching. Since these processes are required for the generation of the germinal center (GC) and antigen-specific plasma and memory cells following immunization with a T-dependent antigen, CB2 has the capacity to alter the quality and magnitude of T-dependent immune responses. To address this question, we immunized WT and CB2(-/-) mice with the T-dependent antigen 4-hydroxy-3-nitrophenylacetyl (NP)-chicken-gamma-globulin (CGG) and measured GC B cell formation and the generation of antigen-specific B cells and serum immunoglobulin (Ig). While there was a significant reduction in the number of splenic GC B cells in CB2(-/-) mice early in the response there was no detectable difference in the number of NP-specific IgM and IgG1 plasma cells. There was also no difference in NP-specific IgM and class switched IgG1 in the serum. In addition, we found no defect in the homing of plasma cells to the bone marrow (BM) and affinity maturation, although memory B cell cells in the spleen were reduced in CB2(-/-) mice. CB2-deficient mice also generated similar levels of antigen-specific IgM and IgG in the serum as WT following immunization with sheep red blood cells (sRBC). This study demonstrates that although CB2 plays a role in promoting GC and memory B cell formation/maintenance in the spleen, it is dispensable on all immune cell types required for the generation of antigen-specific IgM and IgG in T-dependent immune responses.
Conflict of interest statement
Figures
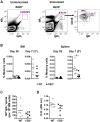
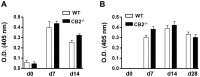
Similar articles
-
Murine complement receptor 1 is required for germinal center B cell maintenance but not initiation.Immunobiology. 2014 Jun;219(6):440-9. doi: 10.1016/j.imbio.2014.02.007. Epub 2014 Feb 25. Immunobiology. 2014. PMID: 24636730 Free PMC article.
-
Phosphatidylcholine as a metabolic cue for determining B cell fate and function.Cell Immunol. 2016 Dec;310:78-88. doi: 10.1016/j.cellimm.2016.08.002. Epub 2016 Aug 3. Cell Immunol. 2016. PMID: 27502364 Free PMC article.
-
Positive selection of IgG+ over IgM+ B cells in the germinal center reaction.Immunity. 2021 May 11;54(5):988-1001.e5. doi: 10.1016/j.immuni.2021.03.013. Epub 2021 Apr 14. Immunity. 2021. PMID: 33857421
-
Why do we need IgM memory B cells?Immunol Lett. 2013 May;152(2):114-20. doi: 10.1016/j.imlet.2013.04.007. Epub 2013 May 6. Immunol Lett. 2013. PMID: 23660557 Review.
-
Human IgM-expressing memory B cells.Front Immunol. 2023 Dec 8;14:1308378. doi: 10.3389/fimmu.2023.1308378. eCollection 2023. Front Immunol. 2023. PMID: 38143767 Free PMC article. Review.
Cited by
-
Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling.Br J Pharmacol. 2014 Dec;171(23):5387-406. doi: 10.1111/bph.12850. Epub 2014 Sep 5. Br J Pharmacol. 2014. PMID: 25048571 Free PMC article.
-
Gut Microbial Dysbiosis Due to Helicobacter Drives an Increase in Marginal Zone B Cells in the Absence of IL-10 Signaling in Macrophages.J Immunol. 2015 Oct 1;195(7):3071-85. doi: 10.4049/jimmunol.1500153. Epub 2015 Aug 31. J Immunol. 2015. PMID: 26324769 Free PMC article.
-
Impact of Δ9-Tetrahydrocannabinol on Rheumatoid Arthritis Synovial Fibroblasts Alone and in Co-Culture with Peripheral Blood Mononuclear Cells.Biomedicines. 2022 May 11;10(5):1118. doi: 10.3390/biomedicines10051118. Biomedicines. 2022. PMID: 35625855 Free PMC article.
-
Cannabinoid receptor 2 engagement promotes group 2 innate lymphoid cell expansion and enhances airway hyperreactivity.J Allergy Clin Immunol. 2022 May;149(5):1628-1642.e10. doi: 10.1016/j.jaci.2021.09.037. Epub 2021 Oct 18. J Allergy Clin Immunol. 2022. PMID: 34673048 Free PMC article.
-
Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal Bacteriophages Administered per os.Front Immunol. 2019 Nov 14;10:2607. doi: 10.3389/fimmu.2019.02607. eCollection 2019. Front Immunol. 2019. PMID: 31803179 Free PMC article.
References
-
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65. - PubMed
-
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, et al. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232: 54–61. - PubMed
-
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, et al. (2000) Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem 275: 605–612. - PubMed
-
- Derocq JM, Segui M, Marchand J, Le Fur G, Casellas P (1995) Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett 369: 177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

