Low resolution structural studies indicate that the activator of Hsp90 ATPase 1 (Aha1) of Leishmania braziliensis has an elongated shape which allows its interaction with both N- and M-domains of Hsp90
- PMID: 23826147
- PMCID: PMC3691308
- DOI: 10.1371/journal.pone.0066822
Low resolution structural studies indicate that the activator of Hsp90 ATPase 1 (Aha1) of Leishmania braziliensis has an elongated shape which allows its interaction with both N- and M-domains of Hsp90
Abstract
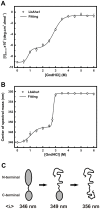
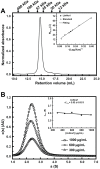
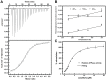
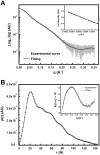
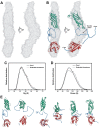
The Hsp90 molecular chaperone is essential for protein homeostasis and in the maturation of proteins involved with cell-cycle control. The low ATPase activity of Hsp90 is critical to drive its functional cycle, which is dependent on the Hsp90 cochaperones. The Activator of Hsp90 ATPase-1 (Aha1) is a protein formed by two domains, N- and C-terminal, that stimulates the Hsp90 ATPase activity by several folds. Although the relevance of Aha1 for Hsp90 functions has been proved, as well as its involvement in the desensitization to inhibitors of the Hsp90, the knowledge on its overall structure and behavior in solution is limited. In this work we present the functional and structural characterization of Leishmania braziliensis Aha1 (LbAha1). This protozoan is the causative agent of cutaneous and mucocutaneous leishmaniasis, a neglected disease. The recombinant LbAha1 behaves as an elongated monomer and is organized into two folded domains interconnected by a flexible linker. Functional experiments showed that LbAha1 interacts with L. braziliensis Hsp90 (LbHsp90) with micromolar dissociation constant in a stoichiometry of 2 LbAha1 to 1 LbHsp90 dimer and stimulates 10-fold the LbHsp90 ATPase activity showing positive cooperativity. Furthermore, the LbHsp90::LbAha1 complex is directed by enthalphy and opposed by entropy, probably due to the spatial freedom restrictions imposed by the proteins' interactions. Small-angle X-ray scattering data allowed the reconstruction of low resolution models and rigid body simulations of LbAha1, indicating its mode of action on LbHsp90. Western blot experiments allowed Aha1 identification (as well as Hsp90) in three Leishmania species at two temperatures, suggesting that Aha1 is a cognate protein. All these data shed light on the LbAha1 mechanism of action, showing that it has structural dimensions and flexibility that allow interacting with both N-terminal and middle domains of the LbHsp90.
Conflict of interest statement
Figures


Similar articles
-
Structural and functional studies of Leishmania braziliensis Hsp90.Biochim Biophys Acta. 2013 Jan;1834(1):351-61. doi: 10.1016/j.bbapap.2012.08.004. Epub 2012 Aug 15. Biochim Biophys Acta. 2013. PMID: 22910377
-
Insights on the structural dynamics of Leishmania braziliensis Hsp90 molecular chaperone by small angle X-ray scattering.Int J Biol Macromol. 2017 Apr;97:503-512. doi: 10.1016/j.ijbiomac.2017.01.058. Epub 2017 Jan 16. Int J Biol Macromol. 2017. PMID: 28104372
-
Low sequence identity but high structural and functional conservation: The case of Hsp70/Hsp90 organizing protein (Hop/Sti1) of Leishmania braziliensis.Arch Biochem Biophys. 2016 Jun 15;600:12-22. doi: 10.1016/j.abb.2016.04.008. Epub 2016 Apr 19. Arch Biochem Biophys. 2016. PMID: 27103305
-
p23 and Aha1: Distinct Functions Promote Client Maturation.Subcell Biochem. 2023;101:159-187. doi: 10.1007/978-3-031-14740-1_6. Subcell Biochem. 2023. PMID: 36520307 Review.
-
Aha-type co-chaperones: the alpha or the omega of the Hsp90 ATPase cycle?Biol Chem. 2020 Mar 26;401(4):423-434. doi: 10.1515/hsz-2019-0341. Biol Chem. 2020. PMID: 31782942 Review.
Cited by
-
Possible Involvement of Hsp90 in the Regulation of Telomere Length and Telomerase Activity During the Leishmania amazonensis Developmental Cycle and Population Proliferation.Front Cell Dev Biol. 2021 Oct 28;9:713415. doi: 10.3389/fcell.2021.713415. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778247 Free PMC article.
-
In silico analysis of the HSP90 chaperone system from the African trypanosome, Trypanosoma brucei.Front Mol Biosci. 2022 Sep 23;9:947078. doi: 10.3389/fmolb.2022.947078. eCollection 2022. Front Mol Biosci. 2022. PMID: 36213128 Free PMC article.
-
Minor temperature shifts do not affect chromosomal ploidy but cause transcriptomic changes in Leishmania braziliensis promastigotes in vitro.Mem Inst Oswaldo Cruz. 2020 Apr 22;115:e190413. doi: 10.1590/0074-02760190413. eCollection 2020. Mem Inst Oswaldo Cruz. 2020. PMID: 32348407 Free PMC article.
-
Molecular Chaperones of Leishmania: Central Players in Many Stress-Related and -Unrelated Physiological Processes.Biomed Res Int. 2015;2015:301326. doi: 10.1155/2015/301326. Epub 2015 Jun 18. Biomed Res Int. 2015. PMID: 26167482 Free PMC article. Review.
-
A review of multi-domain and flexible molecular chaperones studies by small-angle X-ray scattering.Biophys Rev. 2016 Jun;8(2):107-120. doi: 10.1007/s12551-016-0194-x. Epub 2016 Mar 4. Biophys Rev. 2016. PMID: 28510050 Free PMC article. Review.
References
-
- Li J, Soroka J, Buchner J (2012) The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. BBA-Mol Cell Res 1823: 624–635 doi: 10.1016/j.bbamcr.2011.09.003 - DOI - PubMed
-
- Shonhai A, Maier A, Przyborski J, Blatch G (2011) Intracellular Protozoan Parasites of Humans: The Role of Molecular Chaperones in Development and Pathogenesis. Protein Pept Lett 18: 143–157 10.2174/092986611794475002. - PubMed
-
- Pavithra SR, Banumathy G, Joy O, Singh V, Tatu U (2004) Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J Biol Chem 279: 46692–46699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

