Bruton's Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages
- PMID: 23825946
- PMCID: PMC3694848
- DOI: 10.1371/journal.ppat.1003446
Bruton's Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages
Abstract
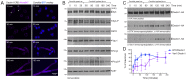
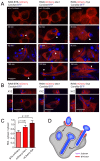
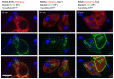
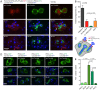
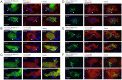
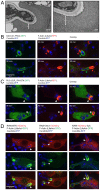
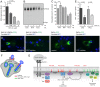
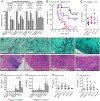
Phagocytosis of the opportunistic fungal pathogen Candida albicans by cells of the innate immune system is vital to prevent infection. Dectin-1 is the major phagocytic receptor involved in anti-fungal immunity. We identify two new interacting proteins of Dectin-1 in macrophages, Bruton's Tyrosine Kinase (BTK) and Vav1. BTK and Vav1 are recruited to phagocytic cups containing C. albicans yeasts or hyphae but are absent from mature phagosomes. BTK and Vav1 localize to cuff regions surrounding the hyphae, while Dectin-1 lines the full length of the phagosome. BTK and Vav1 colocalize with the lipid PI(3,4,5)P3 and F-actin at the phagocytic cup, but not with diacylglycerol (DAG) which marks more mature phagosomal membranes. Using a selective BTK inhibitor, we show that BTK contributes to DAG synthesis at the phagocytic cup and the subsequent recruitment of PKCε. BTK- or Vav1-deficient peritoneal macrophages display a defect in both zymosan and C. albicans phagocytosis. Bone marrow-derived macrophages that lack BTK or Vav1 show reduced uptake of C. albicans, comparable to Dectin1-deficient cells. BTK- or Vav1-deficient mice are more susceptible to systemic C. albicans infection than wild type mice. This work identifies an important role for BTK and Vav1 in immune responses against C. albicans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Intestinal colonization by Candida albicans alters inflammatory responses in Bruton's tyrosine kinase-deficient mice.PLoS One. 2014 Nov 7;9(11):e112472. doi: 10.1371/journal.pone.0112472. eCollection 2014. PLoS One. 2014. PMID: 25379804 Free PMC article.
-
Anion Exchanger 2 Regulates Dectin-1-Dependent Phagocytosis and Killing of Candida albicans.PLoS One. 2016 Jul 8;11(7):e0158893. doi: 10.1371/journal.pone.0158893. eCollection 2016. PLoS One. 2016. PMID: 27391897 Free PMC article.
-
Dual functions of Bruton's tyrosine kinase and Tec kinase during Fcgamma receptor-induced signaling and phagocytosis.J Immunol. 2008 Jul 1;181(1):288-98. doi: 10.4049/jimmunol.181.1.288. J Immunol. 2008. PMID: 18566394
-
Leukotrienes target F-actin/cofilin-1 to enhance alveolar macrophage anti-fungal activity.J Biol Chem. 2011 Aug 19;286(33):28902-28913. doi: 10.1074/jbc.M111.235309. Epub 2011 Jun 29. J Biol Chem. 2011. PMID: 21715328 Free PMC article.
-
CARD9 Syk-dependent and Raf-1 Syk-independent signaling pathways in target recognition of Candida albicans by Dectin-1.Eur J Clin Microbiol Infect Dis. 2011 Mar;30(3):303-5. doi: 10.1007/s10096-010-1103-z. Epub 2010 Nov 25. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21108038 Review.
Cited by
-
Fenebrutinib, a Bruton's tyrosine kinase inhibitor, blocks distinct human microglial signaling pathways.J Neuroinflammation. 2024 Oct 27;21(1):276. doi: 10.1186/s12974-024-03267-5. J Neuroinflammation. 2024. PMID: 39465429 Free PMC article.
-
Hyphal Als proteins act as CR3 ligands to promote immune responses against Candida albicans.Nat Commun. 2024 May 9;15(1):3926. doi: 10.1038/s41467-024-48093-8. Nat Commun. 2024. PMID: 38724513 Free PMC article.
-
BTK inhibitor-induced defects in human neutrophil effector activity against Aspergillus fumigatus are restored by TNF-α.JCI Insight. 2024 May 7;9(12):e176162. doi: 10.1172/jci.insight.176162. JCI Insight. 2024. PMID: 38713531 Free PMC article.
-
Severe Fatal Mucormycosis in a Patient with Chronic Lymphocytic Leukaemia Treated with Zanubrutinib: A Case Report and Review of the Literature.Curr Oncol. 2023 Sep 7;30(9):8255-8265. doi: 10.3390/curroncol30090599. Curr Oncol. 2023. PMID: 37754514 Free PMC article.
-
[Analysis of infection in B-cell lymphoma patients treated with BTK inhibitors].Zhonghua Xue Ye Xue Za Zhi. 2023 Jul 14;44(7):582-586. doi: 10.3760/cma.j.issn.0253-2727.2023.07.011. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37749040 Free PMC article. Chinese. No abstract available.
References
-
- Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, et al. (2004) Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104: 4038–4045. - PubMed
-
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, et al. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22: 507–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

