Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v
- PMID: 23824815
- PMCID: PMC3754103
- DOI: 10.1128/JVI.01038-13
Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v
Abstract
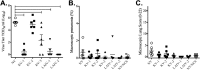
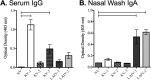
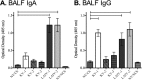
Vaccines provide a primary means to limit disease but may not be effective at blocking infection and pathogen transmission. The objective of the present study was to evaluate the efficacy of commercial inactivated swine influenza A virus (IAV) vaccines and experimental live attenuated influenza virus (LAIV) vaccines against infection with H3N2 virus and subsequent indirect transmission to naive pigs. The H3N2 virus evaluated was similar to the H3N2v detected in humans during 2011-2012, which was associated with swine contact at agricultural fairs. One commercial vaccine provided partial protection measured by reduced nasal shedding; however, indirect contacts became infected, indicating that the reduction in nasal shedding did not prevent aerosol transmission. One LAIV vaccine provided complete protection, and none of the indirect-contact pigs became infected. Clinical disease was not observed in any group, including nonvaccinated animals, a consistent observation in pigs infected with contemporary reassortant H3N2 swine viruses. Serum hemagglutination inhibition antibody titers against the challenge virus were not predictive of efficacy; titers following vaccination with a LAIV that provided sterilizing immunity were below the level considered protective, yet titers in a commercial vaccine group that was not protected were above that level. While vaccination with currently approved commercial inactivated products did not fully prevent transmission, certain vaccines may provide a benefit by limitating shedding, transmission, and zoonotic spillover of antigenically similar H3N2 viruses at agriculture fairs when administered appropriately and used in conjunction with additional control measures.
Figures

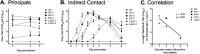
Similar articles
-
Swine influenza virus vaccine serologic cross-reactivity to contemporary US swine H3N2 and efficacy in pigs infected with an H3N2 similar to 2011-2012 H3N2v.Influenza Other Respir Viruses. 2013 Dec;7 Suppl 4(Suppl 4):32-41. doi: 10.1111/irv.12189. Influenza Other Respir Viruses. 2013. PMID: 24224818 Free PMC article.
-
Comparison of Adjuvanted-Whole Inactivated Virus and Live-Attenuated Virus Vaccines against Challenge with Contemporary, Antigenically Distinct H3N2 Influenza A Viruses.J Virol. 2018 Oct 29;92(22):e01323-18. doi: 10.1128/JVI.01323-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185589 Free PMC article.
-
Novel Reassortant Human-Like H3N2 and H3N1 Influenza A Viruses Detected in Pigs Are Virulent and Antigenically Distinct from Swine Viruses Endemic to the United States.J Virol. 2015 Nov;89(22):11213-22. doi: 10.1128/JVI.01675-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311895 Free PMC article.
-
Swine influenza virus vaccines: to change or not to change-that's the question.Curr Top Microbiol Immunol. 2013;370:173-200. doi: 10.1007/82_2012_266. Curr Top Microbiol Immunol. 2013. PMID: 22976350 Review.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
Cited by
-
Pre-exposure with influenza A virus A/WSN/1933(H1N1) resulted in viral shedding reduction from pigs challenged with either swine H1N1 or H3N2 virus.Vet Microbiol. 2019 Jan;228:26-31. doi: 10.1016/j.vetmic.2018.11.008. Epub 2018 Nov 16. Vet Microbiol. 2019. PMID: 30593376 Free PMC article.
-
Detection of influenza A virus in aerosols of vaccinated and non-vaccinated pigs in a warm environment.PLoS One. 2018 May 21;13(5):e0197600. doi: 10.1371/journal.pone.0197600. eCollection 2018. PLoS One. 2018. PMID: 29782527 Free PMC article.
-
Mutation E48K in PB1 Polymerase Subunit Improves Stability of a Candidate Live Attenuated Influenza B Virus Vaccine.Vaccines (Basel). 2021 Jul 19;9(7):800. doi: 10.3390/vaccines9070800. Vaccines (Basel). 2021. PMID: 34358217 Free PMC article.
-
Movement patterns of exhibition swine and associations of influenza A virus infection with swine management practices.J Am Vet Med Assoc. 2017 Sep 15;251(6):706-713. doi: 10.2460/javma.251.6.706. J Am Vet Med Assoc. 2017. PMID: 28857695 Free PMC article.
-
Development of an Alternative Modified Live Influenza B Virus Vaccine.J Virol. 2017 May 26;91(12):e00056-17. doi: 10.1128/JVI.00056-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381580 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) 2011. Swine-origin influenza A (H3N2) virus infection in two children—Indiana and Pennsylvania, July-August 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1213–1215 - PubMed
-
- Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, Juleen K, Simonson R, Brockwell-Staats C, Rubrum A, Wang D, Webb A, Crumpton JC, Lowe J, Gramer M, Webby RJ. 2011. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg. Infect. Dis. 17:1624–1629 - PMC - PubMed
-
- Liu Q, Ma J, Liu H, Qi W, Anderson J, Henry SC, Hesse RA, Richt JA, Ma W. 2012. Emergence of novel reassortant H3N2 swine influenza viruses with the 2009 pandemic H1N1 genes in the United States. Arch. Virol. 157:555–562 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

