Cell susceptibility to baculovirus transduction and echovirus infection is modified by protein kinase C phosphorylation and vimentin organization
- PMID: 23824807
- PMCID: PMC3754104
- DOI: 10.1128/JVI.01004-13
Cell susceptibility to baculovirus transduction and echovirus infection is modified by protein kinase C phosphorylation and vimentin organization
Abstract
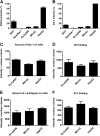
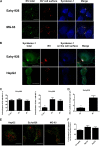
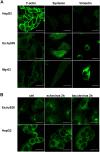
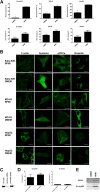
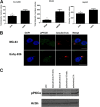
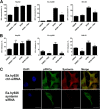
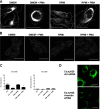
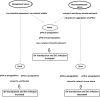
Some cell types are more susceptible to viral gene transfer or virus infection than others, irrespective of the number of viral receptors or virus binding efficacy on their surfaces. In order to characterize the cell-line-specific features contributing to efficient virus entry, we studied two cell lines (Ea.hy926 and MG-63) that are nearly nonpermissive to insect-specific baculovirus (BV) and the human enterovirus echovirus 1 (EV1) and compared their characteristics with those of a highly permissive (HepG2) cell line. All the cell lines contained high levels of viral receptors on their surfaces, and virus binding was shown to be efficient. However, in nonpermissive cells, BV and its receptor, syndecan 1, were unable to internalize in the cells and formed large aggregates near the cell surface. Accordingly, EV1 had a low infection rate in nonpermissive cells but was still able to internalize the cells, suggesting that the postinternalization step of the virus was impaired. The nonpermissive and permissive cell lines showed differential expression of syntenin, filamentous actin, vimentin, and phosphorylated protein kinase C subtype α (pPKCα). The nonpermissive nature of the cells could be modulated by the choice of culture medium. RPMI medium could partially rescue infection/transduction and concomitantly showed lower syntenin expression, a modified vimentin network, and altered activities of PKC subtypes PKCα and PKCε. The observed changes in PKCα and PKCε activation caused alterations in the vimentin organization, leading to efficient BV transduction and EV1 infection. This study identifies PKCα, PKCε, and vimentin as key factors affecting efficient infection and transduction by EV1 and BV, respectively.
Figures
Similar articles
-
Molecular mechanism of alpha2beta1 integrin interaction with human echovirus 1.EMBO J. 2010 Jan 6;29(1):196-208. doi: 10.1038/emboj.2009.326. Epub 2009 Nov 19. EMBO J. 2010. PMID: 19927126 Free PMC article.
-
Cholesterol dependence of collagen and echovirus 1 trafficking along the novel α2β1 integrin internalization pathway.PLoS One. 2013;8(2):e55465. doi: 10.1371/journal.pone.0055465. Epub 2013 Feb 5. PLoS One. 2013. PMID: 23393580 Free PMC article.
-
Echovirus 1 infection depends on biogenesis of novel multivesicular bodies.Cell Microbiol. 2011 Dec;13(12):1975-95. doi: 10.1111/j.1462-5822.2011.01685.x. Epub 2011 Sep 22. Cell Microbiol. 2011. PMID: 21899700
-
Infectious Entry Pathway of Enterovirus B Species.Viruses. 2015 Dec 7;7(12):6387-99. doi: 10.3390/v7122945. Viruses. 2015. PMID: 26690201 Free PMC article. Review.
-
Baculovirus Entry and Egress from Insect Cells.Annu Rev Virol. 2018 Sep 29;5(1):113-139. doi: 10.1146/annurev-virology-092917-043356. Epub 2018 Jul 13. Annu Rev Virol. 2018. PMID: 30004832 Review.
Cited by
-
Baculovirus-mediated gene delivery and RNAi applications.Viruses. 2015 Apr 22;7(4):2099-125. doi: 10.3390/v7042099. Viruses. 2015. PMID: 25912715 Free PMC article. Review.
-
Human Enterovirus Group B Viruses Rely on Vimentin Dynamics for Efficient Processing of Viral Nonstructural Proteins.J Virol. 2020 Jan 6;94(2):e01393-19. doi: 10.1128/JVI.01393-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31619557 Free PMC article.
-
Reply to "heparan sulfate in baculovirus binding and entry of Mammalian cells".J Virol. 2014 Apr;88(8):4609-10. doi: 10.1128/JVI.00083-14. J Virol. 2014. PMID: 24672051 Free PMC article. No abstract available.
-
6-o- and N-sulfated syndecan-1 promotes baculovirus binding and entry into Mammalian cells.J Virol. 2013 Oct;87(20):11148-59. doi: 10.1128/JVI.01919-13. Epub 2013 Aug 7. J Virol. 2013. PMID: 23926339 Free PMC article.
-
Calpain-2 protein influences chikungunya virus replication and regulates vimentin rearrangement caused by chikungunya virus infection.Front Microbiol. 2023 Oct 19;14:1229576. doi: 10.3389/fmicb.2023.1229576. eCollection 2023. Front Microbiol. 2023. PMID: 37928675 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

