Systematic Analysis Reveals Elongation Factor 2 and α-Enolase as Novel Interaction Partners of AKT2
- PMID: 23823123
- PMCID: PMC3688836
- DOI: 10.1371/journal.pone.0066045
Systematic Analysis Reveals Elongation Factor 2 and α-Enolase as Novel Interaction Partners of AKT2
Abstract
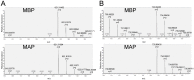
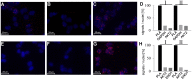
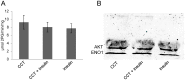
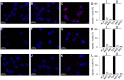
AKT2 is one of the three isoforms of the protein kinase AKT being involved in the modulation of cellular metabolism. Since protein-protein interactions are one possibility to convey specificity in signal transduction, we performed AKT2-protein interaction analysis to elucidate their relevance for AKT2-dependent cellular functions. We identified heat shock protein 90 kDa (HSP90), Cdc37, heat shock protein 70 kDa (HSP70), 78 kDa glucose regulated protein (GRP78), tubulin, GAPDH, α-enolase and elongation factor 2 (EF2) as AKT2-interacting proteins by a combination of tandem affinity purification and mass spectrometry in HEK293T cells. Quantitative MS-analysis using stable isotope labeling by amino acids in cell culture (SILAC) revealed that only HSP90 and Cdc37 interact stably with AKT2, whereas the other proteins interact with low affinity with AKT2. The interactions of AKT2 with α-enolase and EF2 were further analyzed in order to uncover the functional relevance of these newly discovered binding partners. Despite the interaction of AKT2 and α-enolase, which was additionally validated by proximity ligation assay (PLA), no significant impact of AKT on α-enolase activity was detected in activity measurements. AKT stimulation via insulin and/or inhibition with the ATP-competitive inhibitor CCT128930 did not alter enzymatic activity of α-enolase. Interestingly, the direct interaction of AKT2 and EF2 was found to be dynamically regulated in embryonic rat cardiomyocytes. Treatment with the PI3-kinase inhibitor LY294002 before stimulation with several hormones stabilized the complex, whereas stimulation alone led to complex dissociation which was analyzed in situ with PLA. Taken together, these findings point to new aspects of AKT2-mediated signal transduction in protein synthesis and glucose metabolism.
Conflict of interest statement
Figures


Similar articles
-
Novel Endogenous, Insulin-Stimulated Akt2 Protein Interaction Partners in L6 Myoblasts.PLoS One. 2015 Oct 14;10(10):e0140255. doi: 10.1371/journal.pone.0140255. eCollection 2015. PLoS One. 2015. PMID: 26465754 Free PMC article.
-
Identification of elongation factor 1alpha as a potential associated binding partner for Akt2.Mol Cell Biochem. 2006 Jun;286(1-2):17-22. doi: 10.1007/s11010-005-9006-5. Epub 2006 Apr 21. Mol Cell Biochem. 2006. PMID: 16652225
-
Constitutive heat shock protein 70 interacts with α-enolase and protects cardiomyocytes against oxidative stress.Free Radic Res. 2011 Nov;45(11-12):1355-65. doi: 10.3109/10715762.2011.627330. Free Radic Res. 2011. PMID: 21958194
-
α-Enolase, a multifunctional protein: its role on pathophysiological situations.J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. Epub 2012 Oct 14. J Biomed Biotechnol. 2012. PMID: 23118496 Free PMC article. Review.
-
Multifunctional roles of enolase in Alzheimer's disease brain: beyond altered glucose metabolism.J Neurochem. 2009 Nov;111(4):915-33. doi: 10.1111/j.1471-4159.2009.06397.x. Epub 2009 Sep 23. J Neurochem. 2009. PMID: 19780894 Free PMC article. Review.
Cited by
-
Determining the Composition and Stability of Protein Complexes Using an Integrated Label-Free and Stable Isotope Labeling Strategy.Methods Mol Biol. 2016;1410:39-63. doi: 10.1007/978-1-4939-3524-6_3. Methods Mol Biol. 2016. PMID: 26867737 Free PMC article.
-
Defining the Akt1 interactome and its role in regulating the cell cycle.Sci Rep. 2018 Jan 22;8(1):1303. doi: 10.1038/s41598-018-19689-0. Sci Rep. 2018. PMID: 29358593 Free PMC article.
-
Novel Endogenous, Insulin-Stimulated Akt2 Protein Interaction Partners in L6 Myoblasts.PLoS One. 2015 Oct 14;10(10):e0140255. doi: 10.1371/journal.pone.0140255. eCollection 2015. PLoS One. 2015. PMID: 26465754 Free PMC article.
-
Chronic treatment with fluoride affects the jejunum: insights from proteomics and enteric innervation analysis.Sci Rep. 2018 Feb 16;8(1):3180. doi: 10.1038/s41598-018-21533-4. Sci Rep. 2018. PMID: 29453425 Free PMC article.
-
Mitochondrial dysfunction and oxidative stress in aging and cancer.Oncotarget. 2016 Jul 19;7(29):44879-44905. doi: 10.18632/oncotarget.9821. Oncotarget. 2016. PMID: 27270647 Free PMC article. Review.
References
-
- Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596. - PubMed
-
- Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657 10.1038/ncb839. - PubMed
-
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, et al. (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3: 245–252 10.1038/35060032. - PubMed
-
- Datta SR, Dudek H, Tao X, Masters S, Fu H, et al. (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241. - PubMed
-
- Calera MR, Martinez C, Liu H, Jack AK, Birnbaum MJ, et al. (1998) Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem 273: 7201–7204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

