Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2
- PMID: 23821199
- PMCID: PMC3743456
- DOI: 10.1167/iovs.13-11651
Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2
Abstract
Purpose: To determine whether inducing calcification in the trabecular meshwork results in elevated IOP in living rats. To use this property to create an elevated IOP animal model by gene transfer of bone morphogenetic protein 2 (BMP2).
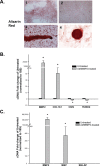
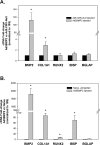
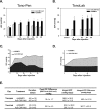
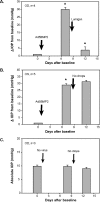
Methods: Calcification was assessed by alizarin red staining in primary human trabecular meshwork (HTM) cells and alkaline phosphatase (ALP) activity in the angle tissue. Brown Norway (BN) and Wistar rats were intracamerally injected with Ad5BMP2 (OS) and control Ad5.CMV-Null (OD). IOPs were taken twice a week and expressed as mean integral pressures. Morphology was assessed on fixed, paraffin-embedded anterior segments. Retinal ganglion cells (RGCs) were quantified on retrograde and Brn-3a-labeled flat mounts using MetaMorph software.
Results: BMP2-treated cells displayed marked increase in calcification. Trabecular meshwork tissue showed moderate ALP activity at 13 days postinjection. Fifty-four of 55 BN and 15 of 19 Wistar rats displayed significantly elevated IOP. In a representative 29-day experiment, the integral IOP difference between treated and control eyes was 367.7 ± 83 mm Hg-days (P = 0.007). Morphological evaluation revealed a well-organized trabecular meshwork tissue, exhibiting denser matrix in the treated eyes. The Ad5BMP2-treated eye showed 34.4% ± 4.8% (P = 0.00002) loss of peripheral RGC over controls.
Conclusions: Gene transfer of the calcification inducer BMP2 gene to the trabecular meshwork induces elevated IOP in living rats without altering the basic structure of the tissue. This strategy generates an elevated IOP model in rats that would be useful for evaluation of glaucoma drugs targeting the outflow pathway.
Keywords: adenoviral gene transfer; elevated IOP model; rat; trabecular meshwork.
Figures


Similar articles
-
Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats.JAMA Ophthalmol. 2015 Feb;133(2):182-90. doi: 10.1001/jamaophthalmol.2014.4747. JAMA Ophthalmol. 2015. PMID: 25412195 Free PMC article.
-
Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma.Invest Ophthalmol Vis Sci. 2015 Jan 13;56(2):893-907. doi: 10.1167/iovs.14-15008. Invest Ophthalmol Vis Sci. 2015. PMID: 25587060 Free PMC article.
-
Nanoencapsulated hybrid compound SA-2 with long-lasting intraocular pressure-lowering activity in rodent eyes.Mol Vis. 2021 Jan 16;27:37-49. eCollection 2021. Mol Vis. 2021. PMID: 33633438 Free PMC article.
-
Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork.Exp Eye Res. 2017 May;158:67-72. doi: 10.1016/j.exer.2016.06.009. Epub 2016 Jun 19. Exp Eye Res. 2017. PMID: 27334250 Free PMC article. Review.
-
Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice.Exp Eye Res. 2013 Nov;116:419-23. doi: 10.1016/j.exer.2013.10.017. Epub 2013 Oct 31. Exp Eye Res. 2013. PMID: 24184030 Free PMC article. Review.
Cited by
-
Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma.Exp Eye Res. 2015 Apr;133:112-25. doi: 10.1016/j.exer.2014.07.014. Exp Eye Res. 2015. PMID: 25819459 Free PMC article. Review.
-
Capsid Mutated Adeno-Associated Virus Delivered to the Anterior Chamber Results in Efficient Transduction of Trabecular Meshwork in Mouse and Rat.PLoS One. 2015 Jun 8;10(6):e0128759. doi: 10.1371/journal.pone.0128759. eCollection 2015. PLoS One. 2015. PMID: 26052939 Free PMC article.
-
A Naturally Fluorescent Mgp Transgenic Mouse for Angiogenesis and Glaucoma Longitudinal Studies.Invest Ophthalmol Vis Sci. 2018 Feb 1;59(2):746-756. doi: 10.1167/iovs.17-22992. Invest Ophthalmol Vis Sci. 2018. PMID: 29392320 Free PMC article.
-
A Comparison of Gene Expression Profiles between Glucocorticoid Responder and Non-Responder Bovine Trabecular Meshwork Cells Using RNA Sequencing.PLoS One. 2017 Jan 9;12(1):e0169671. doi: 10.1371/journal.pone.0169671. eCollection 2017. PLoS One. 2017. PMID: 28068412 Free PMC article.
-
Generation of a Matrix Gla (Mgp) floxed mouse, followed by conditional knockout, uncovers a new Mgp function in the eye.Sci Rep. 2020 Oct 29;10(1):18583. doi: 10.1038/s41598-020-75031-7. Sci Rep. 2020. PMID: 33122788 Free PMC article.
References
-
- Anderson DR. Glaucoma: the damage caused by pressure. XLVI Edward Jackson memorial lecture. Am J Ophthalmol. 1989; 108: 485–495 - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713 - PubMed
-
- Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991; 109: 1090–1095 - PubMed
-
- Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of ‘plaque material' in the inner- and outer wall of Schlemm's canal in normal- and glaucomatous eyes. Exp Eye Res. 1986; 42: 443–455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

