SIRT1 promotes RGC survival and delays loss of function following optic nerve crush
- PMID: 23821198
- PMCID: PMC3726244
- DOI: 10.1167/iovs.13-12157
SIRT1 promotes RGC survival and delays loss of function following optic nerve crush
Abstract
Purpose: Activation of SIRT1 deacetylase prevents retinal ganglion cell (RGC) loss in experimental optic neuritis, an inflammatory optic neuropathy. While mechanisms of this effect are not known, evidence suggests it involves reduction of oxidative stress. We hypothesized that SIRT1 reduces RGC loss due to oxidative stress in noninflammatory optic neuropathies, and examined effects following traumatic injury.
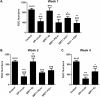
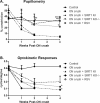
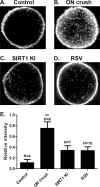
Methods: Optic nerve crush injury was induced in wild-type C57BL/6 mice, mice overexpressing SIRT1, and mice with conditional deletion of SIRT1 in neurons. Wild-type mice were treated daily with vehicle or 250 mg/kg resveratrol, a naturally occurring polyphenol that activates SIRT1. RGC function was assessed by pupillometry and optokinetic responses (OKR), and RGC survival was measured. Superoxide levels were measured to assess oxidative stress.
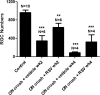
Results: Significant decreases in pupillary light responses, OKR and RGC survival occurred 1 week after optic nerve crush, with progressive worsening at 2 to 4 weeks. Resveratrol treatment and SIRT1 overexpression delayed RGC loss and loss of pupillary light responses following optic nerve crush, although no change in RGC loss occurred in neuronal SIRT1-deficient mice. A significant accumulation of superoxide was detected in wild-type optic nerves following crush, and was reduced in mice overexpressing SIRT1 or treated with resveratrol.
Conclusions: SIRT1 delays RGC loss following traumatic injury. Effects are associated with reduced oxidative stress. Results suggest SIRT1-activating drugs may have a specific role in preventing traumatic optic nerve damage, and suggest a broader role for this strategy in treating a variety of optic neuropathies that may include a component of oxidative stress.
Keywords: SIRT1; neuroprotection; oxidative stress; resveratrol; retinal ganglion cells; traumatic optic neuropathy.
Figures

Similar articles
-
Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1.Gene Ther. 2021 May;28(5):256-264. doi: 10.1038/s41434-021-00219-z. Epub 2021 Feb 15. Gene Ther. 2021. PMID: 33589779 Free PMC article.
-
RGC Neuroprotection Following Optic Nerve Trauma Mediated By Intranasal Delivery of Amnion Cell Secretome.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2470-2477. doi: 10.1167/iovs.18-24096. Invest Ophthalmol Vis Sci. 2018. PMID: 29847652 Free PMC article.
-
Pharmacological Activation and Transgenic Overexpression of SIRT1 Attenuate Traumatic Optic Neuropathy Induced by Blunt Head Impact.Transl Vis Sci Technol. 2024 Sep 3;13(9):27. doi: 10.1167/tvst.13.9.27. Transl Vis Sci Technol. 2024. PMID: 39330985 Free PMC article.
-
Axon Regeneration in the Mammalian Optic Nerve.Annu Rev Vis Sci. 2020 Sep 15;6:195-213. doi: 10.1146/annurev-vision-022720-094953. Annu Rev Vis Sci. 2020. PMID: 32936739 Review.
-
Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration.Antioxidants (Basel). 2021 Dec 5;10(12):1948. doi: 10.3390/antiox10121948. Antioxidants (Basel). 2021. PMID: 34943051 Free PMC article. Review.
Cited by
-
Curcumin and Wnt/β‑catenin signaling in exudative age‑related macular degeneration (Review).Int J Mol Med. 2022 Jun;49(6):79. doi: 10.3892/ijmm.2022.5135. Epub 2022 Apr 21. Int J Mol Med. 2022. PMID: 35445729 Free PMC article. Review.
-
HE3286 reduces axonal loss and preserves retinal ganglion cell function in experimental optic neuritis.Invest Ophthalmol Vis Sci. 2014 Aug 19;55(9):5744-51. doi: 10.1167/iovs.14-14672. Invest Ophthalmol Vis Sci. 2014. PMID: 25139738 Free PMC article.
-
Translational Preclinical Research may Lead to Improved Medical Management of Non-Arteritic Anterior Ischemic Optic Neuropathy.Front Neurol. 2014 Jul 11;5:122. doi: 10.3389/fneur.2014.00122. eCollection 2014. Front Neurol. 2014. PMID: 25071709 Free PMC article. Review. No abstract available.
-
Mitochondrial Open Reading Frame of the 12S rRNA Type-c: Potential Therapeutic Candidate in Retinal Diseases.Antioxidants (Basel). 2023 Feb 18;12(2):518. doi: 10.3390/antiox12020518. Antioxidants (Basel). 2023. PMID: 36830076 Free PMC article. Review.
-
SIRT1 is required for the neuroprotection of resveratrol on retinal ganglion cells after retinal ischemia-reperfusion injury in mice.Graefes Arch Clin Exp Ophthalmol. 2020 Feb;258(2):335-344. doi: 10.1007/s00417-019-04580-z. Epub 2020 Jan 3. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31900639
References
-
- Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005; 26: 94–103 - PubMed
-
- Pallàs M, Casadesús G, Smith MA. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009; 6: 70–81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

