Primary CD8+ T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4+ T cells
- PMID: 23816179
- PMCID: PMC3702406
- DOI: 10.1186/1742-4690-10-68
Primary CD8+ T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4+ T cells
Abstract
Background: Elite controllers or suppressors have the remarkable capacity to maintain HIV-1 plasma RNA levels below the limit of detection of clinical assays (<50 copies/mL) without therapy and have a lower frequency of latently infected cells compared to chronic progressors. While it is unclear how this reduced seeding of the reservoir is achieved, it is possible that effective CTL responses play an in important role in limiting the size of the latent reservoir.
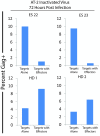
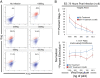
Results: Herein, we demonstrate that primary CD8+ T cells from HLA-B*57/5801 elite suppressors were able to efficiently eliminate resting and activated primary CD4+ T cells shortly after viral entry and prior to productive infection. CD8+ T cells from elite suppressors were significantly more effective at eliminating these cells than CD8+ T cells from chronic progressors.
Conclusions: Nonproductively infected CD4+ T cells may represent a subpopulation of cells that are precursors to latently infected cells; therefore, the effective elimination of these cells may partially explain why elite suppressors have a much lower frequency of latently infected cells compared to chronic progressors. Thus, a vaccine strategy that elicits early and potent CD8+ T cell responses may have the capacity to limit the seeding of the latent reservoir in HIV-1 infection.
Figures



Similar articles
-
Comparative analysis of the capacity of elite suppressor CD4+ and CD8+ T cells to inhibit HIV-1 replication in monocyte-derived macrophages.J Virol. 2014 Sep 1;88(17):9789-98. doi: 10.1128/JVI.00860-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942573 Free PMC article.
-
HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice.J Virol. 2014 Mar;88(6):3340-52. doi: 10.1128/JVI.03380-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390323 Free PMC article.
-
The Effect of Latency Reversal Agents on Primary CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication.EBioMedicine. 2016 Jun;8:217-229. doi: 10.1016/j.ebiom.2016.04.019. Epub 2016 Apr 18. EBioMedicine. 2016. PMID: 27428432 Free PMC article.
-
Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses?Antiviral Res. 2010 Jan;85(1):295-302. doi: 10.1016/j.antiviral.2009.08.007. Epub 2009 Sep 4. Antiviral Res. 2010. PMID: 19733595 Free PMC article. Review.
-
Elucidating the elite: mechanisms of control in HIV-1 infection.Trends Pharmacol Sci. 2009 Dec;30(12):631-7. doi: 10.1016/j.tips.2009.09.005. Trends Pharmacol Sci. 2009. PMID: 19837464 Review.
Cited by
-
Conserved HIV Epitopes for an Effective HIV Vaccine.J Clin Cell Immunol. 2017 Aug;8(4):518. doi: 10.4172/2155-9899.1000518. Epub 2017 Aug 30. J Clin Cell Immunol. 2017. PMID: 29226015 Free PMC article.
-
CD8+ T cells in HIV control, cure and prevention.Nat Rev Immunol. 2020 Aug;20(8):471-482. doi: 10.1038/s41577-020-0274-9. Epub 2020 Feb 12. Nat Rev Immunol. 2020. PMID: 32051540 Free PMC article. Review.
-
The effect of Ingenol-B on the suppressive capacity of elite suppressor HIV-specific CD8+ T cells.PLoS One. 2017 May 3;12(5):e0174516. doi: 10.1371/journal.pone.0174516. eCollection 2017. PLoS One. 2017. PMID: 28467486 Free PMC article.
-
The importance of semen leukocytes in HIV-1 transmission and the development of prevention strategies.Hum Vaccin Immunother. 2020 Sep 1;16(9):2018-2032. doi: 10.1080/21645515.2020.1765622. Epub 2020 Jul 2. Hum Vaccin Immunother. 2020. PMID: 32614649 Free PMC article. Review.
-
More than the Infinite Monkey Theorem: NHP Models in the Development of a Pediatric HIV Cure.Curr HIV/AIDS Rep. 2024 Feb;21(1):11-29. doi: 10.1007/s11904-023-00686-6. Epub 2024 Jan 16. Curr HIV/AIDS Rep. 2024. PMID: 38227162 Free PMC article. Review.
References
-
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. - DOI - PMC - PubMed
-
- Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. - DOI - PMC - PubMed
-
- Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF. SEROCO-HEMOCO Study Group. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41(7):1053–1056. doi: 10.1086/433188. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

