Genetics and Pathophysiology of Neurodegeneration with Brain Iron Accumulation (NBIA)
- PMID: 23814539
- PMCID: PMC3580793
- DOI: 10.2174/157015913804999469
Genetics and Pathophysiology of Neurodegeneration with Brain Iron Accumulation (NBIA)
Abstract
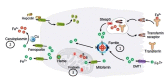
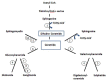
Our understanding of the syndromes of Neurodegeneration with Brain Iron Accumulation (NBIA) continues to grow considerably. In addition to the core syndromes of pantothenate kinase-associated neurodegeneration (PKAN, NBIA1) and PLA2G6-associated neurodegeneration (PLAN, NBIA2), several other genetic causes have been identified (including FA2H, C19orf12, ATP13A2, CP and FTL). In parallel, the clinical and pathological spectrum has broadened and new age-dependent presentations are being described. There is also growing recognition of overlap between the different NBIA disorders and other diseases including spastic paraplegias, leukodystrophies and neuronal ceroid lipofuscinosis which makes a diagnosis solely based on clinical findings challenging. Autopsy examination of genetically-confirmed cases demonstrates Lewy bodies, neurofibrillary tangles, and other hallmarks of apparently distinct neurodegenerative disorders such as Parkinson's disease (PD) and Alzheimer's disease. Until we disentangle the various NBIA genes and their related pathways and move towards pathogenesis-targeted therapies, the treatment remains symptomatic. Our aim here is to provide an overview of historical developments of research into iron metabolism and its relevance in neurodegenerative disorders. We then focus on clinical features and investigational findings in NBIA and summarize therapeutic results reviewing reports of iron chelation therapy and deep brain stimulation. We also discuss genetic and molecular underpinnings of the NBIA syndromes.
Keywords: Ceramide; MPAN; NBIA; PKAN; PLA2G6.; dystonia; iron; parkinsonism.
Figures

Similar articles
-
Excess iron harms the brain: the syndromes of neurodegeneration with brain iron accumulation (NBIA).J Neural Transm (Vienna). 2013 Apr;120(4):695-703. doi: 10.1007/s00702-012-0922-8. Epub 2012 Dec 2. J Neural Transm (Vienna). 2013. PMID: 23212724 Review.
-
Pathophysiology and treatment of neurodegeneration with brain iron accumulation in the pediatric population.Curr Treat Options Neurol. 2013 Oct;15(5):652-67. doi: 10.1007/s11940-013-0254-5. Curr Treat Options Neurol. 2013. PMID: 23888388
-
Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings, and treatment considerations.Mov Disord. 2012 Jan;27(1):42-53. doi: 10.1002/mds.23971. Epub 2011 Oct 26. Mov Disord. 2012. PMID: 22031173 Review.
-
Emerging Disease-Modifying Therapies in Neurodegeneration With Brain Iron Accumulation (NBIA) Disorders.Front Neurol. 2021 Apr 15;12:629414. doi: 10.3389/fneur.2021.629414. eCollection 2021. Front Neurol. 2021. PMID: 33935938 Free PMC article. Review.
-
Review: Insights into molecular mechanisms of disease in neurodegeneration with brain iron accumulation: unifying theories.Neuropathol Appl Neurobiol. 2016 Apr;42(3):220-41. doi: 10.1111/nan.12242. Epub 2015 Jun 2. Neuropathol Appl Neurobiol. 2016. PMID: 25870938 Free PMC article. Review.
Cited by
-
An update on tardive dyskinesia: from phenomenology to treatment.Tremor Other Hyperkinet Mov (N Y). 2013 Jul 12;3:tre-03-161-4138-1. doi: 10.7916/D88P5Z71. Print 2013. Tremor Other Hyperkinet Mov (N Y). 2013. PMID: 23858394 Free PMC article.
-
Utility of susceptibility-weighted imaging in Parkinson's disease and atypical Parkinsonian disorders.Transl Neurodegener. 2016 Oct 7;5:17. doi: 10.1186/s40035-016-0064-2. eCollection 2016. Transl Neurodegener. 2016. PMID: 27761236 Free PMC article. Review.
-
The Interaction of Genetic Mutations in PARK2 and FA2H Causes a Novel Phenotype in a Case of Childhood-Onset Movement Disorder.Front Neurol. 2019 May 29;10:555. doi: 10.3389/fneur.2019.00555. eCollection 2019. Front Neurol. 2019. PMID: 31191442 Free PMC article.
-
Mitochondrial Membrane Protein Associated Neurodegeneration (MPAN) with a Novel C19orf12 Mutation in the First Decade of Life.Indian J Pediatr. 2019 Aug;86(8):746-748. doi: 10.1007/s12098-019-02903-w. Epub 2019 Mar 2. Indian J Pediatr. 2019. PMID: 30825065
-
Transcranial Sonography in Mitochondrial Membrane Protein-Associated Neurodegeneration.Clin Neuroradiol. 2018 Sep;28(3):385-392. doi: 10.1007/s00062-017-0577-9. Epub 2017 Mar 28. Clin Neuroradiol. 2018. PMID: 28352978 Free PMC article.
References
-
- Fleming RE, Ponka P. Iron overload in human disease. N. Engl. J. Med. 2012;366:348–59. - PubMed
-
- Dusek P, Jankovic J, Weidong L. Iron dysregulation in movement disorders. Neurobiol. Dis. 2012;46:1–18. - PubMed
-
- Schneider S A, Hardy J, Bhatia K P. Syndromes of neurodegeneration with brain iron accumulation (NBIA): An update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov. Disord. 2012;27:42–53. - PubMed
-
- Crichton R R, Dexter D T, Ward R J. Brain iron metabolism and its perturbation in neurological diseases. J. Neural. Transm. 2011;118:301–14. - PubMed
-
- Connor J R, Menzies S L, St Martin S M, Mufson E J. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 1990;27:595–611. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
