Prions and the potential transmissibility of protein misfolding diseases
- PMID: 23808331
- PMCID: PMC4784231
- DOI: 10.1146/annurev-micro-092412-155735
Prions and the potential transmissibility of protein misfolding diseases
Abstract
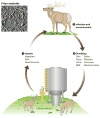
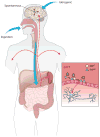
Prions, or infectious proteins, represent a major frontier in the study of infectious agents. The prions responsible for mammalian transmissible spongiform encephalopathies (TSEs) are due primarily to infectious self-propagation of misfolded prion proteins. TSE prion structures remain ill-defined, other than being highly structured, self-propagating, and often fibrillar protein multimers with the capacity to seed, or template, the conversion of their normal monomeric precursors into a pathogenic form. Purified TSE prions usually take the form of amyloid fibrils, which are self-seeding ultrastructures common to many serious protein misfolding diseases such as Alzheimer's, Parkinson's, Huntington's and Lou Gehrig's (amytrophic lateral sclerosis). Indeed, recent reports have now provided evidence of prion-like propagation of several misfolded proteins from cell to cell, if not from tissue to tissue or individual to individual. These findings raise concerns that various protein misfolding diseases might have spreading, prion-like etiologies that contribute to pathogenesis or prevalence.
Figures
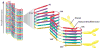
Similar articles
-
[Can prion-like propagation occur in neurodegenerative diseases?: in view of transmissible systemic amyloidosis].Brain Nerve. 2012 Jun;64(6):665-74. Brain Nerve. 2012. PMID: 22647474 Review. Japanese.
-
Misfolded protein aggregates: mechanisms, structures and potential for disease transmission.Semin Cell Dev Biol. 2011 Jul;22(5):482-7. doi: 10.1016/j.semcdb.2011.04.002. Epub 2011 May 5. Semin Cell Dev Biol. 2011. PMID: 21571086 Free PMC article. Review.
-
Animal models for prion-like diseases.Virus Res. 2015 Sep 2;207:5-24. doi: 10.1016/j.virusres.2015.04.014. Epub 2015 Apr 20. Virus Res. 2015. PMID: 25907990 Review.
-
Amyloids, prions and the inherent infectious nature of misfolded protein aggregates.Trends Biochem Sci. 2006 Mar;31(3):150-5. doi: 10.1016/j.tibs.2006.01.002. Epub 2006 Feb 13. Trends Biochem Sci. 2006. PMID: 16473510 Review.
-
The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases.J Cell Biol. 2017 Sep 4;216(9):2633-2644. doi: 10.1083/jcb.201701047. Epub 2017 Jul 19. J Cell Biol. 2017. PMID: 28724527 Free PMC article. Review.
Cited by
-
A Decentralized Approach to the Formulation of Hypotheses: A Hierarchical Structural Model for a Prion Self-Assembled System.Sci Rep. 2016 Jul 28;6:30633. doi: 10.1038/srep30633. Sci Rep. 2016. PMID: 27464832 Free PMC article.
-
Transmissibility versus Pathogenicity of Self-Propagating Protein Aggregates.Viruses. 2019 Nov 9;11(11):1044. doi: 10.3390/v11111044. Viruses. 2019. PMID: 31717531 Free PMC article. Review.
-
Structural biology of ex vivo mammalian prions.J Biol Chem. 2022 Aug;298(8):102181. doi: 10.1016/j.jbc.2022.102181. Epub 2022 Jun 23. J Biol Chem. 2022. PMID: 35752366 Free PMC article. Review.
-
Intrinsic determinants of prion protein neurotoxicity in Drosophila: from sequence to (dys)function.Front Mol Neurosci. 2023 Aug 14;16:1231079. doi: 10.3389/fnmol.2023.1231079. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37645703 Free PMC article. Review.
-
Hermes Transposon Mutagenesis Shows [URE3] Prion Pathology Prevented by a Ubiquitin-Targeting Protein: Evidence for Carbon/Nitrogen Assimilation Cross Talk and a Second Function for Ure2p in Saccharomyces cerevisiae.Genetics. 2018 Jul;209(3):789-800. doi: 10.1534/genetics.118.300981. Epub 2018 May 16. Genetics. 2018. PMID: 29769283 Free PMC article.
References
-
- Agopian A, Guo ZF. Structural origin of polymorphism of Alzheimer’s amyloid beta-fibrils. Biochem J. 2012;447:43–50. - PubMed
-
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–90. - PubMed
-
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. - PubMed
-
- Andronesi OC, von Bergen M, Biernat J, Seidel K, Griesinger C, et al. Characterization of Alzheimer’s-like paired helical filaments from the core domain of tau protein using solid-state NMR spectroscopy. J Am Chem Soc. 2008;130:5922–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

