Understanding vascular development
- PMID: 23799579
- PMCID: PMC4146572
- DOI: 10.1002/wdev.91
Understanding vascular development
Abstract
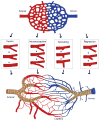
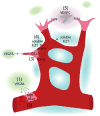
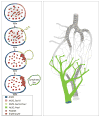
The vasculature of an organism has the daunting task of connecting all the organ systems to nourish tissue and sustain life. This complex network of vessels and associated cells must maintain blood flow, but constantly adapt to acute and chronic changes within tissues. While the vasculature has been studied for over a century, we are just beginning to understand the processes that regulate its formation and how genetic hierarchies are influenced by mechanical and metabolic cues to refine vessel structure and optimize efficiency. As we gain insights into the developmental mechanisms, it is clear that the processes that regulate blood vessel development can also enable the adult to adapt to changes in tissues that can be elicited by exercise, aging, injury, or pathology. Thus, research in vessel development has provided tremendous insights into therapies for vascular diseases and disorders, cancer interventions, wound repair and tissue engineering, and in turn, these models have clearly impacted our understanding of development. Here we provide an overview of the development of the vascular system, highlighting several areas of active investigation and key questions that remain to be answered.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Molecular control of endothelial cell behaviour during blood vessel morphogenesis.Nat Rev Mol Cell Biol. 2011 Aug 23;12(9):551-64. doi: 10.1038/nrm3176. Nat Rev Mol Cell Biol. 2011. PMID: 21860391 Free PMC article. Review.
-
[MOLECULAR AND CELLULAR MECHANISMS OF ANGIOGENESIS IN PHYSIOLOGICAL AND PATHOLOGICAL CONDITIONS].Ross Fiziol Zh Im I M Sechenova. 2017 Feb;103(2):121-37. Ross Fiziol Zh Im I M Sechenova. 2017. PMID: 30199169 Review. Russian.
-
VEGF-directed blood vessel patterning: from cells to organism.Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a006452. doi: 10.1101/cshperspect.a006452. Cold Spring Harb Perspect Med. 2012. PMID: 22951440 Free PMC article. Review.
-
Developmental and pathological angiogenesis.Annu Rev Cell Dev Biol. 2011;27:563-84. doi: 10.1146/annurev-cellbio-092910-154002. Epub 2011 Jul 13. Annu Rev Cell Dev Biol. 2011. PMID: 21756109 Review.
-
Vascular development in early human embryos and in teratomas derived from human embryonic stem cells.Biol Reprod. 2004 Dec;71(6):2029-36. doi: 10.1095/biolreprod.104.031930. Epub 2004 Aug 18. Biol Reprod. 2004. PMID: 15317687
Cited by
-
Guidance Molecules in Vascular Smooth Muscle.Front Physiol. 2018 Sep 19;9:1311. doi: 10.3389/fphys.2018.01311. eCollection 2018. Front Physiol. 2018. PMID: 30283356 Free PMC article. Review.
-
Exploring early human brain development with structural and physiological neuroimaging.Neuroimage. 2019 Feb 15;187:226-254. doi: 10.1016/j.neuroimage.2018.07.041. Epub 2018 Jul 21. Neuroimage. 2019. PMID: 30041061 Free PMC article. Review.
-
Bioprinting Vasculature: Materials, Cells and Emergent Techniques.Materials (Basel). 2019 Aug 23;12(17):2701. doi: 10.3390/ma12172701. Materials (Basel). 2019. PMID: 31450791 Free PMC article. Review.
-
Lymphatic Tissue Engineering and Regeneration.J Biol Eng. 2018 Dec 17;12:32. doi: 10.1186/s13036-018-0122-7. eCollection 2018. J Biol Eng. 2018. PMID: 30564284 Free PMC article. Review.
-
Coalescent angiogenesis-evidence for a novel concept of vascular network maturation.Angiogenesis. 2022 Feb;25(1):35-45. doi: 10.1007/s10456-021-09824-3. Epub 2021 Dec 14. Angiogenesis. 2022. PMID: 34905124 Free PMC article.
References
-
- Serini G, Bussolino F. Common cues in vascular and axon guidance. Physiology (Bethesda) 2004;19:348–354. - PubMed
-
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. - PubMed
-
- Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

