LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo
- PMID: 23798711
- PMCID: PMC3739030
- DOI: 10.1182/blood-2012-12-472308
LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo
Abstract
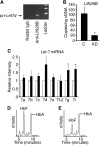
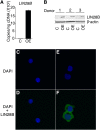
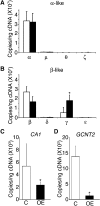
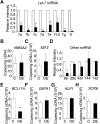
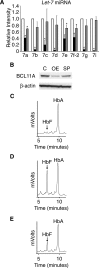
Reactivation of fetal hemoglobin (HbF) holds therapeutic potential for sickle cell disease and β-thalassemias. In human erythroid cells and hematopoietic organs, LIN28B and its targeted let-7 microRNA family, demonstrate regulated expression during the fetal-to-adult developmental transition. To explore the effects of LIN28B in human erythroid cell development, lentiviral transduction was used to knockdown LIN28B expression in erythroblasts cultured from human umbilical cord CD34+ cells. The subsequent reduction in LIN28B expression caused increased expression of let-7 and significantly reduced HbF expression. Conversely, LIN28B overexpression in cultured adult erythroblasts reduced the expression of let-7 and significantly increased HbF expression. Cellular maturation was maintained including enucleation. LIN28B expression in adult erythroblasts increased the expression of γ-globin, and the HbF content of the cells rose to levels >30% of their hemoglobin. Expression of carbonic anhydrase I, glucosaminyl (N-acetyl) transferase 2, and miR-96 (three additional genes marking the transition from fetal-to-adult erythropoiesis) were reduced by LIN28B expression. The transcription factor BCL11A, a well-characterized repressor of γ-globin expression, was significantly down-regulated. Independent of LIN28B, experimental suppression of let-7 also reduced BCL11A expression and significantly increased HbF expression. LIN28B expression regulates HbF levels and causes adult human erythroblasts to differentiate with a more fetal-like phenotype.
Figures


Similar articles
-
HMGA2 Moderately Increases Fetal Hemoglobin Expression in Human Adult Erythroblasts.PLoS One. 2016 Nov 18;11(11):e0166928. doi: 10.1371/journal.pone.0166928. eCollection 2016. PLoS One. 2016. PMID: 27861570 Free PMC article.
-
14q32 and let-7 microRNAs regulate transcriptional networks in fetal and adult human erythroblasts.Hum Mol Genet. 2018 Apr 15;27(8):1411-1420. doi: 10.1093/hmg/ddy051. Hum Mol Genet. 2018. PMID: 29432581 Free PMC article.
-
Tough decoy targeting of predominant let-7 miRNA species in adult human hematopoietic cells.J Transl Med. 2017 Aug 2;15(1):169. doi: 10.1186/s12967-017-1273-x. J Transl Med. 2017. PMID: 28768505 Free PMC article.
-
Reawakening fetal hemoglobin: prospects for new therapies for the β-globin disorders.Blood. 2012 Oct 11;120(15):2945-53. doi: 10.1182/blood-2012-06-292078. Epub 2012 Aug 17. Blood. 2012. PMID: 22904296 Free PMC article. Review.
-
Erythroid enucleation: a gateway into a "bloody" world.Exp Hematol. 2021 Mar;95:13-22. doi: 10.1016/j.exphem.2021.01.001. Epub 2021 Jan 10. Exp Hematol. 2021. PMID: 33440185 Free PMC article. Review.
Cited by
-
Transcriptional Repressor BCL11A in Erythroid Cells.Adv Exp Med Biol. 2024;1459:199-215. doi: 10.1007/978-3-031-62731-6_9. Adv Exp Med Biol. 2024. PMID: 39017845 Review.
-
Modeling primitive and definitive erythropoiesis with induced pluripotent stem cells.Blood Adv. 2024 Mar 26;8(6):1449-1463. doi: 10.1182/bloodadvances.2023011708. Blood Adv. 2024. PMID: 38290102 Free PMC article.
-
Developmental maturation of the hematopoietic system controlled by a Lin28b-let-7-Cbx2 axis.Cell Rep. 2022 Apr 5;39(1):110587. doi: 10.1016/j.celrep.2022.110587. Cell Rep. 2022. PMID: 35385744 Free PMC article.
-
Exploring the crosstalk between long non-coding RNAs and microRNAs to unravel potential prognostic and therapeutic biomarkers in β-thalassemia.Mol Biol Rep. 2022 Jul;49(7):7057-7068. doi: 10.1007/s11033-022-07629-1. Epub 2022 Jun 18. Mol Biol Rep. 2022. PMID: 35717472 Review.
-
Comprehensive Analysis of microRNAs in Human Adult Erythropoiesis.Cells. 2021 Nov 4;10(11):3018. doi: 10.3390/cells10113018. Cells. 2021. PMID: 34831239 Free PMC article.
References
-
- Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307(24):1469–1475. - PubMed
-
- Noguchi CT, Schechter AN. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981;58(6):1057–1068. - PubMed
-
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985;65(1):183–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

