Purinergic signalling and cancer
- PMID: 23797685
- PMCID: PMC3889385
- DOI: 10.1007/s11302-013-9372-5
Purinergic signalling and cancer
Abstract
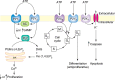
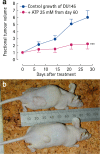
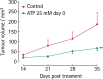
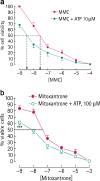
Receptors for extracellular nucleotides are widely expressed by mammalian cells. They mediate a large array of responses ranging from growth stimulation to apoptosis, from chemotaxis to cell differentiation and from nociception to cytokine release, as well as neurotransmission. Pharma industry is involved in the development and clinical testing of drugs selectively targeting the different P1 nucleoside and P2 nucleotide receptor subtypes. As described in detail in the present review, P2 receptors are expressed by all tumours, in some cases to a very high level. Activation or inhibition of selected P2 receptor subtypes brings about cancer cell death or growth inhibition. The field has been largely neglected by current research in oncology, yet the evidence presented in this review, most of which is based on in vitro studies, although with a limited amount from in vivo experiments and human studies, warrants further efforts to explore the therapeutic potential of purinoceptor targeting in cancer.
Figures
Similar articles
-
Introductory overview of purinergic signalling.Front Biosci (Elite Ed). 2011 Jun 1;3(3):896-900. doi: 10.2741/e298. Front Biosci (Elite Ed). 2011. PMID: 21622101 Review.
-
Introduction to Purinergic Signaling.Methods Mol Biol. 2020;2041:1-15. doi: 10.1007/978-1-4939-9717-6_1. Methods Mol Biol. 2020. PMID: 31646477
-
Novel insights into how purines regulate pituitary cell function.Clin Sci (Lond). 2003 May;104(5):467-81. doi: 10.1042/CS20030053. Clin Sci (Lond). 2003. PMID: 12578557 Review.
-
Purinergic signalling--an overview.Novartis Found Symp. 2006;276:26-48; discussion 48-57, 275-81. Novartis Found Symp. 2006. PMID: 16805422 Review.
-
Introduction to purinergic signalling in the brain.Adv Exp Med Biol. 2013;986:1-12. doi: 10.1007/978-94-007-4719-7_1. Adv Exp Med Biol. 2013. PMID: 22879061 Review.
Cited by
-
Prognostic value of purinergic P2X7 receptor expression in patients with hepatocellular carcinoma after curative resection.Tumour Biol. 2015 Jul;36(7):5039-49. doi: 10.1007/s13277-015-3155-2. Epub 2015 Feb 27. Tumour Biol. 2015. PMID: 25722111
-
A2B Adenosine Receptor and Cancer.Int J Mol Sci. 2019 Oct 17;20(20):5139. doi: 10.3390/ijms20205139. Int J Mol Sci. 2019. PMID: 31627281 Free PMC article. Review.
-
Detection of Osmotic Shock-Induced Extracellular Nucleotide Release with a Genetically Encoded Fluorescent Sensor of ADP and ATP.Sensors (Basel). 2019 Jul 24;19(15):3253. doi: 10.3390/s19153253. Sensors (Basel). 2019. PMID: 31344821 Free PMC article.
-
Extracellular ATP and Macropinocytosis: Their Interactive and Mutually Supportive Roles in Cell Growth, Drug Resistance, and EMT in Cancer.Subcell Biochem. 2022;98:61-83. doi: 10.1007/978-3-030-94004-1_4. Subcell Biochem. 2022. PMID: 35378703 Free PMC article. Review.
-
P2Y12 Purinergic Receptor and Brain Tumors: Implications on Glioma Microenvironment.Molecules. 2021 Oct 12;26(20):6146. doi: 10.3390/molecules26206146. Molecules. 2021. PMID: 34684726 Free PMC article. Review.
References
-
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. - PubMed
-
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. - PubMed
-
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. - PubMed
-
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

