CAY10593 inhibits the human P2X7 receptor independently of phospholipase D1 stimulation
- PMID: 23793974
- PMCID: PMC3889394
- DOI: 10.1007/s11302-013-9371-6
CAY10593 inhibits the human P2X7 receptor independently of phospholipase D1 stimulation
Abstract
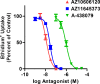
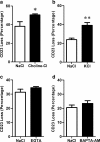
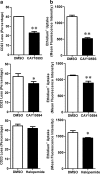
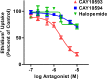
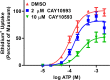
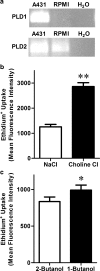
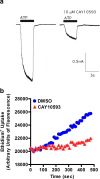
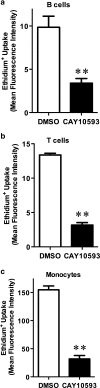
The P2X7 receptor is a trimeric ATP-gated cation channel important in health and disease. We have observed that the specific phospholipase D (PLD)1 antagonist, CAY10593 impairs P2X7-induced shedding of the 'low affinity' IgE receptor, CD23. The current study investigated the mode of action of this compound on P2X7 activation. Measurements of ATP-induced ethidium(+) uptake revealed that CAY10593 impaired P2X7-induced pore formation in human RPMI 8226 B cells, P2X7-transfected HEK-293 cells and peripheral blood mononuclear cells. Concentration response curves demonstrated that CAY10593 impaired P2X7-induced pore formation in RPMI 8226 cells more potently than the PLD2 antagonist CAY10594 and the non-specific PLD antagonist halopemide. Electrophysiology measurements demonstrated that CAY10593 also inhibited P2X7-induced inward currents. Notably, RT-PCR demonstrated that PLD1 was absent in RPMI 8226 cells, while choline-Cl medium or 1-butanol, which block PLD stimulation and signalling respectively did not impair P2X7 activation in these cells. This data indicates that CAY10593 impairs human P2X7 independently of PLD1 stimulation and highlights the importance of ensuring that compounds used in signalling studies downstream of P2X7 activation do not affect the receptor itself.
Figures
Similar articles
-
Probenecid directly impairs activation of the canine P2X7 receptor.Nucleosides Nucleotides Nucleic Acids. 2017 Dec 2;36(12):736-744. doi: 10.1080/15257770.2017.1391395. Epub 2017 Dec 4. Nucleosides Nucleotides Nucleic Acids. 2017. PMID: 29200326
-
P2X7 receptor activation mediates organic cation uptake into human myeloid leukaemic KG-1 cells.Purinergic Signal. 2012 Dec;8(4):669-76. doi: 10.1007/s11302-012-9320-9. Epub 2012 Jun 5. Purinergic Signal. 2012. PMID: 22661222 Free PMC article.
-
Activation of the P2X7 receptor induces the rapid shedding of CD23 from human and murine B cells.Immunol Cell Biol. 2015 Jan;93(1):77-85. doi: 10.1038/icb.2014.69. Epub 2014 Aug 26. Immunol Cell Biol. 2015. PMID: 25155463
-
The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease.Pharmacol Rev. 2014 Jul;66(3):638-75. doi: 10.1124/pr.113.008003. Pharmacol Rev. 2014. PMID: 24928329 Review.
-
[Effect of P2X7 receptor on inflammatory diseases and its mechanism].Sheng Li Xue Bao. 2013 Apr 25;65(2):244-52. Sheng Li Xue Bao. 2013. PMID: 23598883 Review. Chinese.
Cited by
-
6-Furopyridine Hexamethylene Amiloride Is a Non-Selective P2X7 Receptor Antagonist.Biomolecules. 2022 Sep 16;12(9):1309. doi: 10.3390/biom12091309. Biomolecules. 2022. PMID: 36139148 Free PMC article.
-
P2X7 Receptors in Neurodegeneration: Potential Therapeutic Applications From Basic to Clinical Approaches.Front Cell Neurosci. 2021 Apr 6;15:617036. doi: 10.3389/fncel.2021.617036. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33889073 Free PMC article. Review.
-
Probenecid blocks human P2X7 receptor-induced dye uptake via a pannexin-1 independent mechanism.PLoS One. 2014 Mar 26;9(3):e93058. doi: 10.1371/journal.pone.0093058. eCollection 2014. PLoS One. 2014. PMID: 24671093 Free PMC article.
-
The phospholipase D superfamily as therapeutic targets.Trends Pharmacol Sci. 2015 Mar;36(3):137-44. doi: 10.1016/j.tips.2015.01.001. Epub 2015 Feb 3. Trends Pharmacol Sci. 2015. PMID: 25661257 Free PMC article. Review.
-
Cellular and physiological roles for phospholipase D1 in cancer.J Biol Chem. 2014 Aug 15;289(33):22567-22574. doi: 10.1074/jbc.R114.576876. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990946 Free PMC article. Review.
References
-
- Wiley JS, Gargett CE, Zhang W, Snook MB, Jamieson GP. Partial agonists and antagonists reveal a second permeability state of human lymphocyte P2Z/P2X7 channel. Am J Physiol. 1998;275:C1224–C1231. - PubMed
-
- Marques-da-Silva C, Chaves MM, Castro NG, Coutinho-Silva R, Guimaraes MZ. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163:912–926. doi: 10.1111/j.1476-5381.2011.01254.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

