Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice
- PMID: 23788140
- PMCID: PMC3739031
- DOI: 10.1182/blood-2013-03-492504
Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice
Abstract
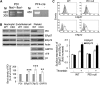
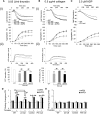
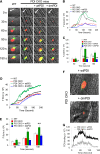
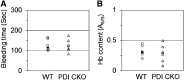
Protein disulfide isomerase (PDI) derived from intravascular cells is required for thrombus formation. However, it remains unclear whether platelet PDI contributes to the process. Using platelet-specific PDI-deficient mice, we demonstrate that PDI-null platelets have defects in aggregation and adenosine triphosphate secretion induced by thrombin, collagen, and adenosine diphosphate. Such defects were rescued by wild-type but not mutant PDI, indicating that the isomerase activity of platelet surface PDI is critical for the regulatory effect. PDI-deficient platelets expressed increased levels of intracellular ER protein 57 (ERp57) and ERp72. Platelet PDI regulated αIIbβ3 integrin activation but not P-selectin exposure, Ca(2+) mobilization, β3-talin1 interaction, or platelet spreading on immobilized fibrinogen. Inhibition of ERp57 further diminished αIIbβ3 integrin activation and aggregation of activated PDI-deficient platelets, suggesting distinct roles of PDI and ERp57 in platelet functions. We found that platelet PDI is important for thrombus formation on collagen-coated surfaces under shear. Intravital microscopy demonstrates that platelet PDI is important for platelet accumulation but not initial adhesion and fibrin generation following laser-induced arteriolar injury. Tail bleeding time in platelet-specific PDI-deficient mice were not significantly increased. Our results provide important evidence that platelet PDI is essential for thrombus formation but not for hemostasis in mice.
Figures



Similar articles
-
Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins.Blood. 2012 Jul 19;120(3):647-55. doi: 10.1182/blood-2011-08-372532. Epub 2012 May 31. Blood. 2012. PMID: 22653978 Free PMC article.
-
Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the αIIbβ3 integrin.Blood. 2013 Nov 21;122(22):3642-50. doi: 10.1182/blood-2013-06-506691. Epub 2013 Sep 12. Blood. 2013. PMID: 24030382 Free PMC article.
-
Multiple protein disulfide isomerases support thrombosis.Curr Opin Hematol. 2018 Sep;25(5):395-402. doi: 10.1097/MOH.0000000000000449. Curr Opin Hematol. 2018. PMID: 29994898 Free PMC article. Review.
-
Platelet-targeting thiol reduction sensor detects thiol isomerase activity on activated platelets in mouse and human blood under flow.J Thromb Haemost. 2016 May;14(5):1070-81. doi: 10.1111/jth.13245. Epub 2016 Mar 7. J Thromb Haemost. 2016. PMID: 26725377 Free PMC article.
-
Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis.J Thromb Haemost. 2024 Jul;22(7):1806-1818. doi: 10.1016/j.jtha.2024.03.008. Epub 2024 Mar 20. J Thromb Haemost. 2024. PMID: 38518897 Review.
Cited by
-
Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis.Blood. 2015 Apr 2;125(14):2276-85. doi: 10.1182/blood-2013-12-547208. Epub 2015 Jan 26. Blood. 2015. PMID: 25624318 Free PMC article.
-
Discovery of 5-Hydroxy-1,4-naphthoquinone (Juglone) Derivatives as Dual Effective Agents Targeting Platelet-Cancer Interplay through Protein Disulfide Isomerase Inhibition.J Med Chem. 2024 Mar 14;67(5):3626-3642. doi: 10.1021/acs.jmedchem.3c02107. Epub 2024 Feb 21. J Med Chem. 2024. PMID: 38381886 Free PMC article.
-
Vascular thiol isomerases in thrombosis: The yin and yang.J Thromb Haemost. 2020 Nov;18(11):2790-2800. doi: 10.1111/jth.15019. Epub 2020 Aug 24. J Thromb Haemost. 2020. PMID: 32702157 Free PMC article. Review.
-
Shear and Integrin Outside-In Signaling Activate NADPH-Oxidase 2 to Promote Platelet Activation.Arterioscler Thromb Vasc Biol. 2021 May 5;41(5):1638-1653. doi: 10.1161/ATVBAHA.120.315773. Epub 2021 Mar 11. Arterioscler Thromb Vasc Biol. 2021. PMID: 33691478 Free PMC article.
-
ERO1-PDI Redox Signaling in Health and Disease.Antioxid Redox Signal. 2021 Nov 1;35(13):1093-1115. doi: 10.1089/ars.2021.0018. Epub 2021 Jul 13. Antioxid Redox Signal. 2021. PMID: 34074138 Free PMC article. Review.
References
-
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. - PubMed
-
- Zucker MB, Masiello NC. Platelet aggregation caused by dithiothreitol. Thromb Haemost. 1984;51(1):119–124. - PubMed
-
- Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273(14):7981–7987. - PubMed
-
- O’Neill S, Robinson A, Deering A, Ryan M, Fitzgerald DJ, Moran N. The platelet integrin alpha IIbbeta 3 has an endogenous thiol isomerase activity. J Biol Chem. 2000;275(47):36984–36990. - PubMed
-
- Yan B, Smith JW. A redox site involved in integrin activation. J Biol Chem. 2000;275(51):39964–39972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

