Msb1 interacts with Cdc42, Boi1, and Boi2 and may coordinate Cdc42 and Rho1 functions during early stage of bud development in budding yeast
- PMID: 23785492
- PMCID: PMC3681933
- DOI: 10.1371/journal.pone.0066321
Msb1 interacts with Cdc42, Boi1, and Boi2 and may coordinate Cdc42 and Rho1 functions during early stage of bud development in budding yeast
Abstract
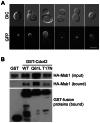
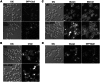
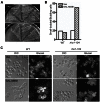
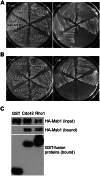
Msb1 is not essential for growth in the budding yeast Saccharomyces cerevisiae since msb1Δ cells do not display obvious phenotypes. Genetic studies suggest that Msb1 positively regulates Cdc42 function during bud development, since high-copy MSB1 suppressed the growth defect of temperature-sensitive cdc24 and cdc42 mutants at restrictive temperature, while deletion of MSB1 showed synthetic lethality with cdc24, bem1, and bem2 mutations. However, the mechanism of how Msb1 regulates Cdc42 function remains poorly understood. Here, we show that Msb1 localizes to sites of polarized growth during bud development and interacts with Cdc42 in the cells. In addition, Msb1 interacts with Boi1 and Boi2, two scaffold proteins that also interact with Cdc42 and Bem1. These findings suggest that Msb1 may positively regulate Cdc42 function by interacting with Cdc42, Boi1, and Boi2, which may promote the efficient assembly of Cdc42, Cdc24, and other proteins into a functional complex. We also show that Msb1 interacts with Rho1 in the cells and Msb1 overproduction inhibits the growth of rho1-104 and rho1-3 but not rho1-2 cells. The growth inhibition appears to result from the down-regulation of Rho1 function in glucan synthesis, specifically during early stage of bud development. These results suggest that Msb1 may coordinate Cdc42 and Rho1 functions during early stage of bud development by promoting Cdc42 function and inhibiting Rho1 function. Msb1 overproduction also affects cell morphology, septin organization, and causes increased, aberrant deposition of 1,3-β-glucan and chitin at the mother-bud neck. However, the stimulation of glucan synthesis mainly occurs during late, but not early, stage of bud development.
Conflict of interest statement
Figures


Similar articles
-
Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae.Mol Cell Biol. 1991 Mar;11(3):1295-305. doi: 10.1128/mcb.11.3.1295-1305.1991. Mol Cell Biol. 1991. PMID: 1996092 Free PMC article.
-
Gbetagamma recruits Rho1 to the site of polarized growth during mating in budding yeast.J Biol Chem. 2003 Jun 13;278(24):21798-804. doi: 10.1074/jbc.M212636200. Epub 2003 Mar 26. J Biol Chem. 2003. PMID: 12660244
-
Roles of the PH, coiled-coil and SAM domains of the yeast polarity protein Boi2 in polarity-site localization and function in polarized growth.Curr Genet. 2020 Dec;66(6):1101-1115. doi: 10.1007/s00294-020-01093-9. Epub 2020 Jul 12. Curr Genet. 2020. PMID: 32656574
-
Role of small G proteins in yeast cell polarization and wall biosynthesis.Annu Rev Biochem. 1998;67:307-33. doi: 10.1146/annurev.biochem.67.1.307. Annu Rev Biochem. 1998. PMID: 9759491 Free PMC article. Review.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
-
The APSES transcription factor Vst1 is a key regulator of development in microsclerotium- and resting mycelium-producing Verticillium species.Mol Plant Pathol. 2018 Jan;19(1):59-76. doi: 10.1111/mpp.12496. Epub 2017 Jan 13. Mol Plant Pathol. 2018. PMID: 27696683 Free PMC article.
-
Complex Haploinsufficiency-Based Genetic Analysis of the NDR/Lats Kinase Cbk1 Provides Insight into Its Multiple Functions in Candida albicans.Genetics. 2016 Jul;203(3):1217-33. doi: 10.1534/genetics.116.188029. Epub 2016 May 20. Genetics. 2016. PMID: 27206715 Free PMC article.
-
Evolutionary dynamics in the fungal polarization network, a mechanistic perspective.Biophys Rev. 2017 Aug;9(4):375-387. doi: 10.1007/s12551-017-0286-2. Epub 2017 Aug 15. Biophys Rev. 2017. PMID: 28812259 Free PMC article. Review.
-
Regulation of intrinsic polarity establishment by a differentiation-type MAPK pathway in S. cerevisiae.J Cell Sci. 2020 Apr 14;133(7):jcs241513. doi: 10.1242/jcs.241513. J Cell Sci. 2020. PMID: 32079658 Free PMC article.
-
Generation and characterization of conditional yeast mutants affecting each of the 2 essential functions of the scaffolding proteins Boi1/2 and Bem1.G3 (Bethesda). 2022 Dec 1;12(12):jkac273. doi: 10.1093/g3journal/jkac273. G3 (Bethesda). 2022. PMID: 36218417 Free PMC article.
References
-
- Barral Y, Mermall V, Mooseker MS, Snyder M (2000) Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell 5: 841–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

