The adjuvant activity of alphavirus replicons is enhanced by incorporating the microbial molecule flagellin into the replicon
- PMID: 23785460
- PMCID: PMC3681802
- DOI: 10.1371/journal.pone.0065964
The adjuvant activity of alphavirus replicons is enhanced by incorporating the microbial molecule flagellin into the replicon
Abstract
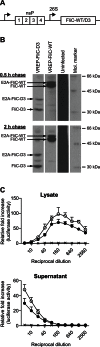
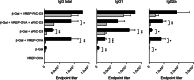
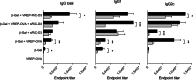
Ligands of pattern recognition receptors (PRRs) including Toll-like receptors (TLRs) stimulate innate and adaptive immune responses and are considered as potent adjuvants. Combinations of ligands might act in synergy to induce stronger and broader immune responses compared to stand-alone ligands. Alphaviruses stimulate endosomal TLRs 3, 7 and 8 as well as the cytoplasmic PRR MDA-5, resulting in induction of a strong type I interferon (IFN) response. Bacterial flagellin stimulates TLR5 and when delivered intracellularly the cytosolic PRR NLRC4, leading to secretion of proinflammatory cytokines. Both alphaviruses and flagellin have independently been shown to act as adjuvants for antigen-specific antibody responses. Here, we hypothesized that alphavirus and flagellin would act in synergy when combined. We therefore cloned the Salmonella Typhimurium flagellin (FliC) gene into an alphavirus replicon and assessed its adjuvant activity on the antibody response against co-administered antigen. In mice immunized with recombinant alphavirus, antibody responses were greatly enhanced compared to soluble FliC or control alphavirus. Both IgG1 and IgG2a/c responses were increased, indicating an enhancement of both Th1 and Th2 type responses. The adjuvant activity of FliC-expressing alphavirus was diminished but not abolished in the absence of TLR5 or type I IFN signaling, suggesting the contribution of several signaling pathways and some synergistic and redundant activity of its components. Thus, we have created a recombinant adjuvant that stimulates multiple signaling pathways of innate immunity resulting in a strong and broad antibody response.
Conflict of interest statement
Figures


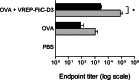

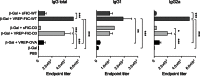
Similar articles
-
Flagellin induces antibody responses through a TLR5- and inflammasome-independent pathway.J Immunol. 2014 Feb 15;192(4):1587-96. doi: 10.4049/jimmunol.1301893. Epub 2014 Jan 17. J Immunol. 2014. PMID: 24442437 Free PMC article.
-
Immunogenic properties of a recombinant fusion protein containing the C-terminal 19 kDa of Plasmodium falciparum merozoite surface protein-1 and the innate immunity agonist FliC flagellin of Salmonella typhimurium.Vaccine. 2010 Apr 1;28(16):2818-26. doi: 10.1016/j.vaccine.2010.02.004. Epub 2010 Feb 17. Vaccine. 2010. PMID: 20170765
-
Adaptive Immunity Induces Tolerance to Flagellin by Attenuating TLR5 and NLRC4-Mediated Innate Immune Responses.Front Cell Infect Microbiol. 2019 Feb 19;9:29. doi: 10.3389/fcimb.2019.00029. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30838179 Free PMC article.
-
Flagellin as a vaccine adjuvant.Expert Rev Vaccines. 2018 Apr;17(4):335-349. doi: 10.1080/14760584.2018.1457443. Epub 2018 Mar 30. Expert Rev Vaccines. 2018. PMID: 29580106 Review.
-
Linking genetic variation in human Toll-like receptor 5 genes to the gut microbiome's potential to cause inflammation.Immunol Lett. 2014 Dec;162(2 Pt A):3-9. doi: 10.1016/j.imlet.2014.07.017. Epub 2014 Oct 2. Immunol Lett. 2014. PMID: 25284610 Free PMC article. Review.
Cited by
-
From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children.Int J Mol Sci. 2023 Feb 3;24(3):3001. doi: 10.3390/ijms24033001. Int J Mol Sci. 2023. PMID: 36769320 Free PMC article. Review.
-
De Novo Transcriptome Analysis Shows That SAV-3 Infection Upregulates Pattern Recognition Receptors of the Endosomal Toll-Like and RIG-I-Like Receptor Signaling Pathways in Macrophage/Dendritic Like TO-Cells.Viruses. 2016 Apr 21;8(4):114. doi: 10.3390/v8040114. Viruses. 2016. PMID: 27110808 Free PMC article.
-
DNA Vaccine-Encoded Flagellin Can Be Used as an Adjuvant Scaffold to Augment HIV-1 gp41 Membrane Proximal External Region Immunogenicity.Viruses. 2018 Feb 27;10(3):100. doi: 10.3390/v10030100. Viruses. 2018. PMID: 29495537 Free PMC article.
-
Alphavirus replicon DNA expressing HIV antigens is an excellent prime for boosting with recombinant modified vaccinia Ankara (MVA) or with HIV gp140 protein antigen.PLoS One. 2015 Feb 2;10(2):e0117042. doi: 10.1371/journal.pone.0117042. eCollection 2015. PLoS One. 2015. PMID: 25643354 Free PMC article.
-
Inflammasomes and adaptive immune responses.Nat Immunol. 2021 Apr;22(4):412-422. doi: 10.1038/s41590-021-00869-6. Epub 2021 Feb 18. Nat Immunol. 2021. PMID: 33603227 Review.
References
-
- Pasare C, Medzhitov R (2005) Control of B-cell responses by Toll-like receptors. Nature 438: 364–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

