A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons
- PMID: 23785143
- PMCID: PMC6618599
- DOI: 10.1523/JNEUROSCI.5048-12.2013
A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons
Abstract
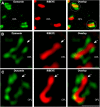
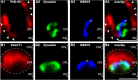
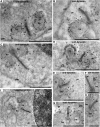
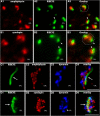
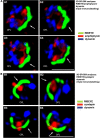
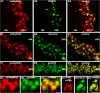
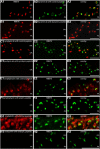
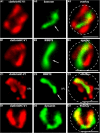
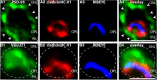
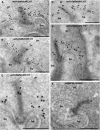
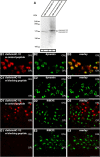
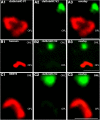
Photoreceptor ribbon synapses are continuously active synapses with large active zones that contain synaptic ribbons. Synaptic ribbons are anchored to the active zones and are associated with large numbers of synaptic vesicles. The base of the ribbon that is located close to L-type voltage-gated Ca(2+) channels is a hotspot of exocytosis. The continuous exocytosis at the ribbon synapse needs to be balanced by compensatory endocytosis. Recent analyses indicated that vesicle recycling at the synaptic ribbon is also an important determinant of synaptic signaling at the photoreceptor synapse. To get insights into mechanisms of vesicle recycling at the photoreceptor ribbon synapse, we performed super-resolution structured illumination microscopy and immunogold electron microscopy to localize major components of the endocytotic membrane retrieval machinery in the photoreceptor synapse of the mouse retina. We found dynamin, syndapin, amphiphysin, and calcineurin, a regulator of activity-dependent endocytosis, to be highly enriched around the active zone and the synaptic ribbon. We present evidence for two clathrin heavy chain variants in the photoreceptor terminal; one is enriched around the synaptic ribbon, whereas the other is localized in the entry region of the terminal. The focal enrichment of endocytic proteins around the synaptic ribbon is consistent with a focal uptake of endocytic markers at that site. This endocytic activity functionally depends on dynamin. These data propose that the presynaptic periactive zone surrounding the synaptic ribbon complex is a hotspot of endocytosis in photoreceptor ribbon synapses.
Figures


Similar articles
-
ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis.J Neurosci. 2014 Apr 9;34(15):5245-60. doi: 10.1523/JNEUROSCI.3837-13.2014. J Neurosci. 2014. PMID: 24719103 Free PMC article.
-
Functional Roles of Complexin 3 and Complexin 4 at Mouse Photoreceptor Ribbon Synapses.J Neurosci. 2016 Jun 22;36(25):6651-67. doi: 10.1523/JNEUROSCI.4335-15.2016. J Neurosci. 2016. PMID: 27335398 Free PMC article.
-
Preferential localization of type I phosphatidylinositol 4-phosphate 5-kinase γ at the periactive zone of mouse photoreceptor ribbon synapses.Brain Res. 2014 Oct 24;1586:23-33. doi: 10.1016/j.brainres.2014.08.051. Epub 2014 Aug 22. Brain Res. 2014. PMID: 25152467
-
The making of synaptic ribbons: how they are built and what they do.Neuroscientist. 2009 Dec;15(6):611-24. doi: 10.1177/1073858409340253. Neuroscientist. 2009. PMID: 19700740 Review.
-
Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses.Front Mol Neurosci. 2014 Feb 6;7:3. doi: 10.3389/fnmol.2014.00003. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24567702 Free PMC article. Review.
Cited by
-
Analytical Post-Embedding Immunogold-Electron Microscopy with Direct Gold-Labelled Monoclonal Primary Antibodies against RIBEYE A- and B-Domain Suggests a Refined Model of Synaptic Ribbon Assembly.Int J Mol Sci. 2024 Jul 6;25(13):7443. doi: 10.3390/ijms25137443. Int J Mol Sci. 2024. PMID: 39000549 Free PMC article.
-
Disruption of adaptor protein 2μ (AP-2μ) in cochlear hair cells impairs vesicle reloading of synaptic release sites and hearing.EMBO J. 2015 Nov 3;34(21):2686-702. doi: 10.15252/embj.201591885. Epub 2015 Oct 7. EMBO J. 2015. PMID: 26446278 Free PMC article.
-
Barriers to the free diffusion of proteins and lipids in the plasma membrane.J Cell Biol. 2015 Feb 2;208(3):259-71. doi: 10.1083/jcb.201410071. J Cell Biol. 2015. PMID: 25646084 Free PMC article. Review.
-
Phosphatidylserine and GTPase activation control Cdc42 nanoclustering to counter dissipative diffusion.Mol Biol Cell. 2018 Jun 1;29(11):1299-1310. doi: 10.1091/mbc.E18-01-0051. Epub 2018 Apr 18. Mol Biol Cell. 2018. PMID: 29668348 Free PMC article.
-
A new probe for super-resolution imaging of membranes elucidates trafficking pathways.J Cell Biol. 2014 May 26;205(4):591-606. doi: 10.1083/jcb.201402066. J Cell Biol. 2014. PMID: 24862576 Free PMC article.
References
-
- Alpadi K, Magupalli VG, Käppel S, Köblitz L, Schwarz K, Seigel GM, Sung CH, Schmitz F. RIBEYE recruits Munc119, the mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J Biol Chem. 2008;283:26461–26467. doi: 10.1074/jbc.M801625200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
