Overexpression of Arachis hypogaea AREB1 gene enhances drought tolerance by modulating ROS scavenging and maintaining endogenous ABA content
- PMID: 23783278
- PMCID: PMC3709814
- DOI: 10.3390/ijms140612827
Overexpression of Arachis hypogaea AREB1 gene enhances drought tolerance by modulating ROS scavenging and maintaining endogenous ABA content
Abstract
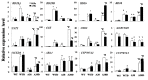
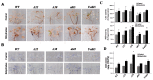
AhAREB1 (Arachis hypogaea Abscisic-acid Response Element Binding Protein 1) is a member of the basic domain leucine zipper (bZIP)-type transcription factor in peanut. Previously, we found that expression of AhAREB1 was specifically induced by abscisic acid (ABA), dehydration and drought. To understand the drought defense mechanism regulated by AhAREB1, transgenic Arabidopsis overexpressing AhAREB1 was conducted in wild-type (WT), and a complementation experiment was employed to ABA non-sensitivity mutant abi5 (abscisic acid-insensitive 5). Constitutive expression of AhAREB1 confers water stress tolerance and is highly sensitive to exogenous ABA. Microarray and further real-time PCR analysis revealed that drought stress, reactive oxygen species (ROS) scavenging, ABA synthesis/metabolism-related genes and others were regulated in transgenic Arabidopsis overexpressing AhAREB1. Accordingly, low level of ROS, but higher ABA content was detected in the transgenic Arabidopsis plants' overexpression of AhAREB1. Taken together, it was concluded that AhAREB1 modulates ROS accumulation and endogenous ABA level to improve drought tolerance in transgenic Arabidopsis.
Figures

Similar articles
-
Negative feedback regulation of ABA biosynthesis in peanut (Arachis hypogaea): a transcription factor complex inhibits AhNCED1 expression during water stress.Sci Rep. 2016 Nov 28;6:37943. doi: 10.1038/srep37943. Sci Rep. 2016. PMID: 27892506 Free PMC article.
-
Expression of AhATL1, an ABA Transport Factor Gene from Peanut, Is Affected by Altered Memory Gene Expression Patterns and Increased Tolerance to Drought Stress in Arabidopsis.Int J Mol Sci. 2021 Mar 25;22(7):3398. doi: 10.3390/ijms22073398. Int J Mol Sci. 2021. PMID: 33806243 Free PMC article.
-
TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat.Plant J. 2022 Nov;112(3):722-737. doi: 10.1111/tpj.15975. Epub 2022 Sep 27. Plant J. 2022. PMID: 36097863
-
Drought coping strategies in cotton: increased crop per drop.Plant Biotechnol J. 2017 Mar;15(3):271-284. doi: 10.1111/pbi.12688. Plant Biotechnol J. 2017. PMID: 28055133 Free PMC article. Review.
-
Persistence of Abscisic Acid Analogs in Plants: Chemical Control of Plant Growth and Physiology.Genes (Basel). 2023 May 13;14(5):1078. doi: 10.3390/genes14051078. Genes (Basel). 2023. PMID: 37239437 Free PMC article. Review.
Cited by
-
Unraveling the induction of phytoene synthase 2 expression by salt stress and abscisic acid in Daucus carota.J Exp Bot. 2018 Jul 18;69(16):4113-4126. doi: 10.1093/jxb/ery207. J Exp Bot. 2018. PMID: 29860511 Free PMC article.
-
Engineering drought tolerance in plants through CRISPR/Cas genome editing.3 Biotech. 2020 Sep;10(9):400. doi: 10.1007/s13205-020-02390-3. Epub 2020 Aug 19. 3 Biotech. 2020. PMID: 32864285 Free PMC article. Review.
-
Overexpression of LcSABP, an Orthologous Gene for Salicylic Acid Binding Protein 2, Enhances Drought Stress Tolerance in Transgenic Tobacco.Front Plant Sci. 2019 Feb 21;10:200. doi: 10.3389/fpls.2019.00200. eCollection 2019. Front Plant Sci. 2019. PMID: 30847000 Free PMC article.
-
Increasing yield on dry fields: molecular pathways with growing potential.Plant J. 2022 Jan;109(2):323-341. doi: 10.1111/tpj.15550. Epub 2021 Nov 8. Plant J. 2022. PMID: 34695266 Free PMC article. Review.
-
6-SFT, a Protein from Leymus mollis, Positively Regulates Salinity Tolerance and Enhances Fructan Levels in Arabidopsis thaliana.Int J Mol Sci. 2019 May 31;20(11):2691. doi: 10.3390/ijms20112691. Int J Mol Sci. 2019. PMID: 31159261 Free PMC article.
References
-
- Wan X.R., Li L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochem. Biophys. Res. Commun. 2006;347:1030–1038. - PubMed
-
- Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. - PubMed
-
- Seki M., Umezawa T., Urano K., Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007;10:296–302. - PubMed
-
- Sreenivasulu N., Harshavardhan V.T., Govind G., Seiler C., Kohli A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene. 2012;506:265–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

