Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke
- PMID: 23781399
- PMCID: PMC3684213
- DOI: 10.1089/ther.2013.0010
Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke
Abstract
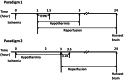
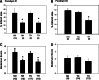
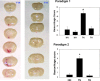
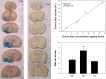
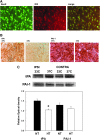
Therapeutic hypothermia has shown neuroprotective promise, but whether it can be used to improve outcome in stroke has yet to be determined in patients. Recombinant tissue plasminogen activator (rt-PA) is only given to a minority of patients with acute ischemic stroke, and is not without risk, namely significant brain hemorrhage.We explored whether mild hypothermia, in combination with rt-PA, influences the safety of rt-PA. Mice were subjected to middle cerebral artery occlusion (MCAO) using a filament model, followed by 24 hours reperfusion.Two paradigms were studied. In the first paradigm, cooling and rt-PA treatment began at the same time upon reperfusion, whereas in the second paradigm, cooling began soon after ischemia onset, and rt-PA began after rewarming and upon reperfusion. Experimental groups included: tPA treatment at normothermia (37°C), rt-PA treatment at hypothermia (33°C), no rt-PA at normothermia, and no rt-PA treatment at hypothermia. Infarct size, neurological deficit scores, blood brain barrier (BBB) permeability, brain hemorrhage, and expression of endogenous tissue plasminogen activator (tPA) and its inhibitor, plasminogen activator inhibitor (PAI-1) were assessed. For both paradigms, hypothermia reduced infarct size and neurological deficits compared to normothermia, regardless of whether rt-PA was given. rt-PA treatment increased brain hemorrhage and BBB disruption compared to normothermia, and this was prevented by cooling. However, mortality was higher when rt-PA and cooling were administered at the same time, beginning 1–2 hours post MCAO. Endogenous tPA expression was reduced in hypothermic mice, whereas PAI-1 levels were unchanged by cooling. In the setting of rt-PA treatment, hypothermia reduces brain hemorrhage, and BBB disruption, suggesting that combination therapy with mild hypothermia and rt-PA appears safe.
Figures
Similar articles
-
Transient brain hypothermia reduces the reperfusion injury of delayed tissue plasminogen activator and extends its therapeutic time window in a focal embolic stroke model.Brain Res Bull. 2017 Sep;134:85-90. doi: 10.1016/j.brainresbull.2017.07.007. Epub 2017 Jul 11. Brain Res Bull. 2017. PMID: 28710023
-
LDL receptor blockade reduces mortality in a mouse model of ischaemic stroke without improving tissue-type plasminogen activator-induced brain haemorrhage: towards pre-clinical simulation of symptomatic ICH.Fluids Barriers CNS. 2017 Nov 21;14(1):33. doi: 10.1186/s12987-017-0081-2. Fluids Barriers CNS. 2017. PMID: 29157263 Free PMC article.
-
Adjuvant therapies using normobaric oxygen with hypothermia or ethanol for reducing hyperglycolysis in thromboembolic cerebral ischemia.Neuroscience. 2016 Mar 24;318:45-57. doi: 10.1016/j.neuroscience.2016.01.010. Epub 2016 Jan 12. Neuroscience. 2016. PMID: 26794589
-
Combination therapy with hypothermia for treatment of cerebral ischemia.J Neurotrauma. 2009 Mar;26(3):325-31. doi: 10.1089/neu.2008.0594. J Neurotrauma. 2009. PMID: 19216635 Free PMC article. Review.
-
Oxygen or cooling, to make a decision after acute ischemia stroke.Med Gas Res. 2016 Dec 30;6(4):206-211. doi: 10.4103/2045-9912.196902. eCollection 2016 Oct-Dec. Med Gas Res. 2016. PMID: 28217292 Free PMC article. Review.
Cited by
-
Role of scalp hypothermia in patients undergoing minimally invasive evacuation of hypertensive cerebral hemorrhage.Pak J Med Sci. 2019 Sep-Oct;35(5):1451-1455. doi: 10.12669/pjms.35.5.593. Pak J Med Sci. 2019. PMID: 31489024 Free PMC article.
-
Recombinant Tissue Plasminogen Activator Induces Neurological Side Effects Independent on Thrombolysis in Mechanical Animal Models of Focal Cerebral Infarction: A Systematic Review and Meta-Analysis.PLoS One. 2016 Jul 7;11(7):e0158848. doi: 10.1371/journal.pone.0158848. eCollection 2016. PLoS One. 2016. PMID: 27387385 Free PMC article. Review.
-
Clinical perspectives on ischemic stroke.Exp Neurol. 2021 Apr;338:113599. doi: 10.1016/j.expneurol.2021.113599. Epub 2021 Jan 10. Exp Neurol. 2021. PMID: 33440204 Free PMC article. Review.
-
Selective Brain Cooling: A New Horizon of Neuroprotection.Front Neurol. 2022 Jun 20;13:873165. doi: 10.3389/fneur.2022.873165. eCollection 2022. Front Neurol. 2022. PMID: 35795804 Free PMC article. Review.
-
Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise.Neuropharmacology. 2018 May 15;134(Pt B):302-309. doi: 10.1016/j.neuropharm.2017.08.025. Epub 2017 Aug 19. Neuropharmacology. 2018. PMID: 28830757 Free PMC article. Review.
References
-
- Aoki T. Sumii T. Mori T. Wang X. Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. - PubMed
-
- Asahi M. Asahi K. Jung JC. del Zoppo GJ. Fini ME. Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. - PubMed
-
- Bederson JB. Pitts LH. Tsuji M. Nishimura MC. Davis RL. Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
-
- Belayev L. Busto R. Zhao W. Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. - PubMed
-
- Bernard SA. Gray TW. Buist MD. Jones BM. Silvester W. Gutteridge G. Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

