β1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27
- PMID: 23780416
- PMCID: PMC3695668
- DOI: 10.1007/s00232-013-9571-6
β1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27
Abstract
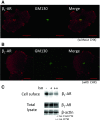
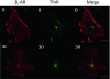
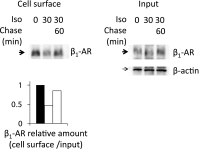
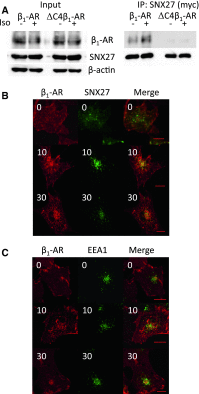
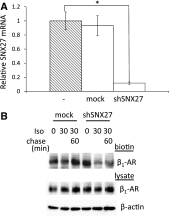
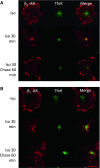
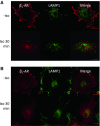
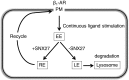
In cardiomyocytes, β1-adrenergic receptor (β1-AR) plays an important role in regulating cardiac functions. Upon continuous ligand stimulation, β1-AR is internalized and mostly recycled back to the plasma membrane (PM). The recycling endosome (RE) is one of the membranous organelles involved in the protein recycling pathway. To determine whether RE is involved in the internalization of β1-AR upon ligand stimulation, we evaluated the localization of β1-AR after stimulation with a β-agonist, isoproterenol (Iso), in β1-AR-transfected COS-1 cells. After 30 min of Iso treatment and cell surface labeling with the appropriate antibodies, β1-AR was internalized from PM and translocated into the perinuclear region, the same location as the transferrin receptor, an RE marker. We then evaluated whether sorting nexin 27 (SNX27) participated in the β1-AR recycling pathway. When β1-AR and SNX27 were coexpressed, β1-AR coimmunoprecipitated with SNX27. In addition, shRNA-mediated silencing of SNX27 compromised β1-AR recycling and enhanced its delivery into lysosome. Overall, β1-AR on PM was internalized into RE upon Iso stimulation and recycled by RE through binding with SNX27 in COS-1 cells.
Figures
Similar articles
-
Identification of novel transplantable GPCR recycling motif for drug discovery.Biochem Pharmacol. 2016 Nov 15;120:22-32. doi: 10.1016/j.bcp.2016.09.011. Epub 2016 Sep 16. Biochem Pharmacol. 2016. PMID: 27645110 Free PMC article.
-
Two barcodes encoded by the type-1 PDZ and by phospho-Ser312 regulate retromer/WASH-mediated sorting of the ß1-adrenergic receptor from endosomes to the plasma membrane.Cell Signal. 2017 Jan;29:192-208. doi: 10.1016/j.cellsig.2016.10.014. Epub 2016 Nov 2. Cell Signal. 2017. PMID: 27816670
-
Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling.J Biol Chem. 2016 May 20;291(21):10986-1002. doi: 10.1074/jbc.M115.697045. Epub 2016 Mar 23. J Biol Chem. 2016. PMID: 27008860 Free PMC article.
-
Sorting Nexin 27 as a potential target in G protein‑coupled receptor recycling for cancer therapy (Review).Oncol Rep. 2020 Nov;44(5):1779-1786. doi: 10.3892/or.2020.7766. Epub 2020 Sep 14. Oncol Rep. 2020. PMID: 33000258 Free PMC article. Review.
-
GPCR Signaling and Trafficking: The Long and Short of It.Trends Endocrinol Metab. 2017 Mar;28(3):213-226. doi: 10.1016/j.tem.2016.10.007. Epub 2016 Nov 23. Trends Endocrinol Metab. 2017. PMID: 27889227 Free PMC article. Review.
Cited by
-
Crosstalk between PI3K and Ras pathways via protein phosphatase 2A in human ovarian clear cell carcinoma.Cancer Biol Ther. 2015;16(2):325-35. doi: 10.1080/15384047.2014.1002362. Cancer Biol Ther. 2015. PMID: 25756515 Free PMC article.
-
Minireview: Role of intracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling.Mol Endocrinol. 2015 Jun;29(6):814-30. doi: 10.1210/me.2015-1091. Epub 2015 May 5. Mol Endocrinol. 2015. PMID: 25942107 Free PMC article. Review.
-
Endosomal Sorting Protein SNX27 and Its Emerging Roles in Human Cancers.Cancers (Basel). 2022 Dec 22;15(1):70. doi: 10.3390/cancers15010070. Cancers (Basel). 2022. PMID: 36612066 Free PMC article. Review.
-
Deletion of sorting nexin 27 suppresses proliferation in highly aggressive breast cancer MDA-MB-231 cells in vitro and in vivo.BMC Cancer. 2019 Jun 10;19(1):555. doi: 10.1186/s12885-019-5769-z. BMC Cancer. 2019. PMID: 31182056 Free PMC article.
-
Papillomaviruses and Endocytic Trafficking.Int J Mol Sci. 2018 Sep 4;19(9):2619. doi: 10.3390/ijms19092619. Int J Mol Sci. 2018. PMID: 30181457 Free PMC article. Review.
References
-
- Balana B, Maslennikov I, Kwiatkowski W, Stern KM, Bahima L, Choe S, Slesinger PA. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc Natl Acad Sci USA. 2010;108:5831–5836. doi: 10.1073/pnas.1018645108. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

