Antibody responses in humans infected with newly emerging strains of West Nile Virus in Europe
- PMID: 23776680
- PMCID: PMC3680493
- DOI: 10.1371/journal.pone.0066507
Antibody responses in humans infected with newly emerging strains of West Nile Virus in Europe
Abstract
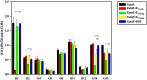
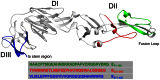
Infection with West Nile Virus (WNV) affects an increasing number of countries worldwide. Although most human infections result in no or mild flu-like symptoms, the elderly and those with a weakened immune system are at higher risk for developing severe neurological disease. Since its introduction into North America in 1999, WNV has spread across the continental United States and caused annual outbreaks with a total of 36,000 documented clinical cases and ∼1,500 deaths. In recent years, outbreaks of neuroinvasive disease also have been reported in Europe. The WNV strains isolated during these outbreaks differ from those in North America, as sequencing has revealed that distinct phylogenetic lineages of WNV concurrently circulate in Europe, which has potential implications for the development of vaccines, therapeutics, and diagnostic tests. Here, we studied the human antibody response to European WNV strains responsible for outbreaks in Italy and Greece in 2010, caused by lineage 1 and 2 strains, respectively. The WNV structural proteins were expressed as a series of overlapping fragments fused to a carrier-protein, and binding of IgG in sera from infected persons was analyzed. The results demonstrate that, although the humoral immune response to WNV in humans is heterogeneous, several dominant peptides are recognized.
Conflict of interest statement
Figures
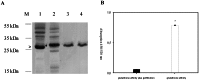
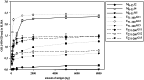
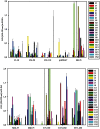
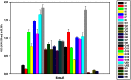
Similar articles
-
West Nile virus: the complex biology of an emerging pathogen.Intervirology. 2011;54(4):171-84. doi: 10.1159/000328320. Epub 2011 May 16. Intervirology. 2011. PMID: 21576931 Review.
-
Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: analysis of human outbreaks in Italy and Greece, 2013-2014.Clin Microbiol Infect. 2015 Dec;21(12):1122.e1-10. doi: 10.1016/j.cmi.2015.07.018. Epub 2015 Jul 31. Clin Microbiol Infect. 2015. PMID: 26235197
-
Laboratory and clinical characteristics of human West Nile virus infections during 2011 outbreak in southern Greece.Vector Borne Zoonotic Dis. 2014 Jan;14(1):52-8. doi: 10.1089/vbz.2013.1369. Epub 2013 Dec 20. Vector Borne Zoonotic Dis. 2014. PMID: 24359426
-
Development time of IgG antibodies to West Nile virus.Arch Virol. 2011 Sep;156(9):1661-3. doi: 10.1007/s00705-011-1014-z. Epub 2011 May 11. Arch Virol. 2011. PMID: 21559972
-
Latest developments and challenges in the diagnosis of human West Nile virus infection.Expert Rev Anti Infect Ther. 2015 Mar;13(3):327-42. doi: 10.1586/14787210.2015.1007044. Epub 2015 Feb 2. Expert Rev Anti Infect Ther. 2015. PMID: 25641365 Review.
Cited by
-
The Japanese Encephalitis Antigenic Complex Viruses: From Structure to Immunity.Viruses. 2022 Oct 8;14(10):2213. doi: 10.3390/v14102213. Viruses. 2022. PMID: 36298768 Free PMC article. Review.
-
Advanced oxidation technology for the development of a next-generation inactivated West Nile virus vaccine.Vaccine. 2019 Jul 9;37(30):4214-4221. doi: 10.1016/j.vaccine.2018.12.020. Epub 2018 Dec 31. Vaccine. 2019. PMID: 30606462 Free PMC article.
-
Identification of Influenza A/H7N9 virus infection-related human genes based on shortest paths in a virus-human protein interaction network.Biomed Res Int. 2014;2014:239462. doi: 10.1155/2014/239462. Epub 2014 May 18. Biomed Res Int. 2014. PMID: 24955349 Free PMC article.
-
Broad and long-lasting immune protection against various Chikungunya genotypes demonstrated by participants in a cross-sectional study in a Cambodian rural community.Emerg Microbes Infect. 2018 Feb 7;7(1):13. doi: 10.1038/s41426-017-0010-0. Emerg Microbes Infect. 2018. PMID: 29410416 Free PMC article.
-
T cell epitope mapping of the e-protein of West Nile virus in BALB/c mice.PLoS One. 2014 Dec 15;9(12):e115343. doi: 10.1371/journal.pone.0115343. eCollection 2014. PLoS One. 2014. PMID: 25506689 Free PMC article.
References
-
- Barzon L, Franchin E, Squarzon L, Lavezzo E, Toppo S, et al... (2009) Genome sequence analysis of the first human West Nile virus isolated in Italy in 2009. Euro Surveill;14. - PubMed
-
- Sirbu A, Ceianu CS, Panculescu-Gatej RI, Vazquez A, Tenorio A, et al... (2010) Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill 2011;16 - PubMed
-
- Barzon L, Pacenti M, Franchin E, Lavezzo E, Martello T, et al... (2012) New endemic West Nile virus lineage 1a in northern Italy, July 2012. Euro Surveill : 17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

