SNARE proteins are essential in the potentiation of NMDA receptors by group II metabotropic glutamate receptors
- PMID: 23774277
- PMCID: PMC3764638
- DOI: 10.1113/jphysiol.2013.255075
SNARE proteins are essential in the potentiation of NMDA receptors by group II metabotropic glutamate receptors
Abstract
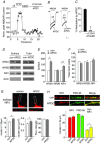
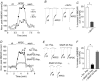
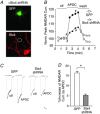
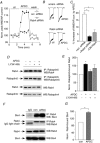
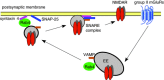
The group II metabotropic glutamate receptors (group II mGluRs) have emerged as the new drug targets for the treatment of mental disorders like schizophrenia. To understand the potential mechanisms underlying the antipsychotic effects of group II mGluRs, we examined their impact on NMDA receptors (NMDARs), since NMDAR hypofunction has been implicated in schizophrenia. The activation of group II mGluRs caused a significant enhancement of NMDAR currents in cortical pyramidal neurons, which was associated with increased NMDAR surface expression and synaptic localization. We further examined whether these effects of group II mGluRs are through the regulation of NMDAR exocytosis via SNARE proteins, a family of proteins involved in vesicle fusion. We found that the enhancing effect of APDC, a selective agonist of group II mGluRs, on NMDAR currents was abolished when botulinum toxin was delivered into the recorded neurons to disrupt the SNARE complex. Inhibiting the function of two key SNARE proteins, SNAP-25 and syntaxin 4, also eliminated the effect of APDC on NMDAR currents. Moreover, the application of APDC increased the activity of Rab4, a small Rab GTPase mediating fast recycling from early endosomes to the plasma membrane, and enhanced the interaction between syntaxin 4 and Rab4. Knockdown of Rab4 or expression of dominant-negative Rab4 attenuated the effect of APDC on NMDAR currents. Taken together, these results have identified key molecules involved in the group II mGluR-induced potentiation of NMDAR exocytosis and function.
Figures
Similar articles
-
Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex.J Physiol. 2004 Feb 1;554(Pt 3):765-77. doi: 10.1113/jphysiol.2003.056812. Epub 2003 Nov 28. J Physiol. 2004. PMID: 14645456 Free PMC article.
-
Evidence for involvement of group II/III metabotropic glutamate receptors in NMDA receptor-independent long-term potentiation in area CA1 of rat hippocampus.J Neurophysiol. 1999 Dec;82(6):2956-69. doi: 10.1152/jn.1999.82.6.2956. J Neurophysiol. 1999. PMID: 10601432
-
beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons.J Neurophysiol. 2005 Jun;93(6):3102-11. doi: 10.1152/jn.00939.2004. Epub 2005 Jan 19. J Neurophysiol. 2005. PMID: 15659527
-
N-methyl-D-aspartate receptor hypofunction as a potential contributor to the progression and manifestation of many neurological disorders.Front Mol Neurosci. 2023 Jun 15;16:1174738. doi: 10.3389/fnmol.2023.1174738. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37396784 Free PMC article. Review.
-
The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia.Front Pharmacol. 2014 Jan 2;4:169. doi: 10.3389/fphar.2013.00169. Front Pharmacol. 2014. PMID: 24427139 Free PMC article. Review.
Cited by
-
The Use of Botulinum Toxin for the Treatment of Chronic Joint Pain: Clinical and Experimental Evidence.Toxins (Basel). 2020 May 10;12(5):314. doi: 10.3390/toxins12050314. Toxins (Basel). 2020. PMID: 32397671 Free PMC article. Review.
-
Targeting metabotropic glutamate receptors for novel treatments of schizophrenia.Mol Brain. 2017 Apr 26;10(1):15. doi: 10.1186/s13041-017-0293-z. Mol Brain. 2017. PMID: 28446243 Free PMC article. Review.
-
Dysregulations of Synaptic Vesicle Trafficking in Schizophrenia.Curr Psychiatry Rep. 2016 Aug;18(8):77. doi: 10.1007/s11920-016-0710-5. Curr Psychiatry Rep. 2016. PMID: 27371030 Free PMC article. Review.
-
Recent progress in understanding subtype specific regulation of NMDA receptors by G Protein Coupled Receptors (GPCRs).Int J Mol Sci. 2014 Feb 20;15(2):3003-24. doi: 10.3390/ijms15023003. Int J Mol Sci. 2014. PMID: 24562329 Free PMC article. Review.
-
SNARE-Mediated Exocytosis in Neuronal Development.Front Mol Neurosci. 2020 Aug 7;13:133. doi: 10.3389/fnmol.2020.00133. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32848598 Free PMC article. Review.
References
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29:83–120. - PubMed
-
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
-
- Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosc Biobehav Rev. 2008;32:1014–1023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

