A new reporter mouse cytomegalovirus reveals maintained immediate-early gene expression but poor virus replication in cycling liver sinusoidal endothelial cells
- PMID: 23773211
- PMCID: PMC3765632
- DOI: 10.1186/1743-422X-10-197
A new reporter mouse cytomegalovirus reveals maintained immediate-early gene expression but poor virus replication in cycling liver sinusoidal endothelial cells
Abstract
Background: The MCMV major immediate early promoter/enhancer (MIEP) is a bidirectional promoter that drives the expression of the three immediate early viral genes, namely ie1, ie2 and ie3. The regulation of their expression is intensively studied, but still incompletely understood.
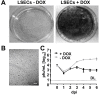
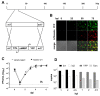
Methods: We constructed a reporter MCMV, (MCMV-MIEPr) expressing YFP and tdTomato under the control of the MIEP as proxies of ie1 and ie2, respectively. Moreover, we generated a liver sinusoidal endothelial cell line (LSEC-uniLT) where cycling is dependent on doxycycline. We used these novel tools to study the kinetics of MIEP-driven gene expression in the context of infection and at the single cell level by flow cytometry and by live imaging of proliferating and G0-arrested cells.
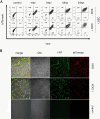
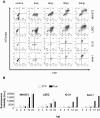
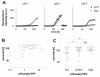
Results: MCMV replicated to higher titers in G0-arrested LSEC, and cycling cells showed less cytopathic effect or YFP and tdTomato expression at 5 days post infection. In the first 24 h post infection, however, there was no difference in MIEP activity in cycling or G0-arrested cells, although we could observe different profiles of MIEP gene expression in different cell types, like LSECs, fibroblasts or macrophages. We monitored infected LSEC-uniLT in G0 by time lapse microscopy over five days and noticed that most cells survived infection for at least 96 h, arguing that quick lysis of infected cells could not account for the spread of the virus. Interestingly, we noticed a strong correlation between the ratio of median YFP and tdTomato expression and length of survival of infected cells.
Conclusion: By means of our newly developed genetic tools, we showed that the expression pattern of MCMV IE1 and IE2 genes differs between macrophages, endothelial cells and fibroblasts. Substantial and cell-cycle independent differences in the ie1 and ie2 transcription could also be observed within individual cells of the same population, and marked ie2 gene expression was associated with longer survival of the infected cells.
Figures
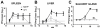
Similar articles
-
The major immediate-early gene ie3 of mouse cytomegalovirus is essential for viral growth.J Virol. 2000 Dec;74(23):11129-36. doi: 10.1128/jvi.74.23.11129-11136.2000. J Virol. 2000. PMID: 11070009 Free PMC article.
-
The Canonical Immediate Early 3 Gene Product pIE611 of Mouse Cytomegalovirus Is Dispensable for Viral Replication but Mediates Transcriptional and Posttranscriptional Regulation of Viral Gene Products.J Virol. 2015 Aug;89(16):8590-8. doi: 10.1128/JVI.01234-15. Epub 2015 Jun 10. J Virol. 2015. PMID: 26063418 Free PMC article.
-
Elimination of ie1 significantly attenuates murine cytomegalovirus virulence but does not alter replicative capacity in cell culture.J Virol. 2005 Jun;79(11):7182-94. doi: 10.1128/JVI.79.11.7182-7194.2005. J Virol. 2005. PMID: 15890957 Free PMC article.
-
Transactivation of cellular genes involved in nucleotide metabolism by the regulatory IE1 protein of murine cytomegalovirus is not critical for viral replicative fitness in quiescent cells and host tissues.J Virol. 2008 Oct;82(20):9900-16. doi: 10.1128/JVI.00928-08. Epub 2008 Aug 6. J Virol. 2008. PMID: 18684825 Free PMC article.
-
Differences between mouse and human cytomegalovirus interactions with their respective hosts at immediate early times of the replication cycle.Med Microbiol Immunol. 2008 Jun;197(2):241-9. doi: 10.1007/s00430-008-0078-1. Epub 2008 Feb 9. Med Microbiol Immunol. 2008. PMID: 18264718 Review.
Cited by
-
The M25 gene products are critical for the cytopathic effect of mouse cytomegalovirus.Sci Rep. 2017 Nov 14;7(1):15588. doi: 10.1038/s41598-017-15783-x. Sci Rep. 2017. PMID: 29138436 Free PMC article.
-
Reversible silencing of cytomegalovirus genomes by type I interferon governs virus latency.PLoS Pathog. 2014 Feb 20;10(2):e1003962. doi: 10.1371/journal.ppat.1003962. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24586165 Free PMC article.
-
Cytomegalovirus immune evasion of myeloid lineage cells.Med Microbiol Immunol. 2015 Jun;204(3):367-82. doi: 10.1007/s00430-015-0403-4. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776081 Review.
-
Cytomegalovirus inhibition of extrinsic apoptosis determines fitness and resistance to cytotoxic CD8 T cells.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12961-12968. doi: 10.1073/pnas.1914667117. Epub 2020 May 22. Proc Natl Acad Sci U S A. 2020. PMID: 32444487 Free PMC article.
-
Type I Interferon Released by Myeloid Dendritic Cells Reversibly Impairs Cytomegalovirus Replication by Inhibiting Immediate Early Gene Expression.J Virol. 2015 Oct;89(19):9886-95. doi: 10.1128/JVI.01459-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202227 Free PMC article.
References
-
- Marcinowski L, Lidschreiber M, Windhager L, Rieder M, Bosse JB, Radle B, Bonfert T, Gyory I, De Graaf M, Prazeres Da Costa O, Rosenstiel P, Friedel CC, Zimmer R, Ruzsics Z, Dolken L. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathogens. 2012;8:e1002908. doi: 10.1371/journal.ppat.1002908. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

