Adipose tissue angiogenesis: impact on obesity and type-2 diabetes
- PMID: 23770388
- PMCID: PMC3844681
- DOI: 10.1016/j.bbadis.2013.06.003
Adipose tissue angiogenesis: impact on obesity and type-2 diabetes
Abstract
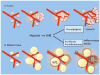
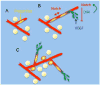
The growth and function of tissues are critically dependent on their vascularization. Adipose tissue is capable of expanding many-fold during adulthood, therefore requiring the formation of new vasculature to supply growing and proliferating adipocytes. The expansion of the vasculature in adipose tissue occurs through angiogenesis, where new blood vessels develop from those pre-existing within the tissue. Inappropriate angiogenesis may underlie adipose tissue dysfunction in obesity, which in turn increases type-2 diabetes risk. In addition, genetic and developmental factors involved in vascular patterning may define the size and expandability of diverse adipose tissue depots, which are also associated with type-2 diabetes risk. Moreover, the adipose tissue vasculature appears to be the niche for pre-adipocyte precursors, and factors that affect angiogenesis may directly impact the generation of new adipocytes. Here we review recent advances on the basic mechanisms of angiogenesis, and on the role of angiogenesis in adipose tissue development and obesity. A substantial amount of data points to a deficit in adipose tissue angiogenesis as a contributing factor to insulin resistance and metabolic disease in obesity. These emerging findings support the concept of the adipose tissue vasculature as a source of new targets for metabolic disease therapies. This article is part of a Special Issue entitled: Modulation of Adipose Tissue in Health and Disease.
Keywords: Adipocyte; Blood vessel; Capillary; Endothelial; Fat; Vascular.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Redox implications in adipose tissue (dys)function--A new look at old acquaintances.Redox Biol. 2015 Dec;6:19-32. doi: 10.1016/j.redox.2015.06.018. Epub 2015 Jul 2. Redox Biol. 2015. PMID: 26177468 Free PMC article. Review.
-
Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues.Diabetologia. 2016 Jun;59(6):1075-88. doi: 10.1007/s00125-016-3933-4. Epub 2016 Apr 4. Diabetologia. 2016. PMID: 27039901 Free PMC article. Review.
-
Angiogenesis in adipose tissue and obesity.Angiogenesis. 2022 Nov;25(4):439-453. doi: 10.1007/s10456-022-09848-3. Epub 2022 Jul 20. Angiogenesis. 2022. PMID: 35857195 Free PMC article. Review.
-
Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity.Physiol Rev. 2018 Oct 1;98(4):1911-1941. doi: 10.1152/physrev.00034.2017. Physiol Rev. 2018. PMID: 30067159 Review.
-
Angiogenesis in obesity.Biomed Pharmacother. 2020 Jun;126:110103. doi: 10.1016/j.biopha.2020.110103. Epub 2020 Mar 19. Biomed Pharmacother. 2020. PMID: 32200253 Review.
Cited by
-
Metabolic pathways in obesity-related breast cancer.Nat Rev Endocrinol. 2021 Jun;17(6):350-363. doi: 10.1038/s41574-021-00487-0. Epub 2021 Apr 29. Nat Rev Endocrinol. 2021. PMID: 33927368 Free PMC article. Review.
-
Plasma Placental Growth Factor Concentrations Are Elevated Well in Advance of Type 2 Diabetes Mellitus Onset: Prospective Data From the WHS.J Am Heart Assoc. 2019 Aug 6;8(15):e012790. doi: 10.1161/JAHA.119.012790. Epub 2019 Jul 19. J Am Heart Assoc. 2019. PMID: 31322059 Free PMC article.
-
The Unexplored Role of Intra-articular Adipose Tissue in the Homeostasis and Pathology of Articular Joints.Front Vet Sci. 2018 Mar 5;5:35. doi: 10.3389/fvets.2018.00035. eCollection 2018. Front Vet Sci. 2018. PMID: 29556503 Free PMC article.
-
The Interactions of Obesity, Inflammation and Insulin Resistance in Breast Cancer.Cancers (Basel). 2015 Oct 26;7(4):2147-68. doi: 10.3390/cancers7040883. Cancers (Basel). 2015. PMID: 26516917 Free PMC article. Review.
-
Endothelial angiogenic activity and adipose angiogenesis is controlled by extracellular matrix protein TGFBI.Sci Rep. 2021 May 6;11(1):9644. doi: 10.1038/s41598-021-88959-1. Sci Rep. 2021. PMID: 33958649 Free PMC article.
References
-
- Nakagami T, Qiao Q, Carstensen B, Nhr-Hansen C, Hu G, Tuomilehto J, Balkau B, Borch-Johnsen K. Age, body mass index and Type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46:1063–1070. - PubMed
-
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

