Impact of anti-CD25 monoclonal antibody on dendritic cell-tumor fusion vaccine efficacy in a murine melanoma model
- PMID: 23768240
- PMCID: PMC3691646
- DOI: 10.1186/1479-5876-11-148
Impact of anti-CD25 monoclonal antibody on dendritic cell-tumor fusion vaccine efficacy in a murine melanoma model
Abstract
Background: A promising cancer vaccine involves the fusion of tumor cells with dendritic cells (DCs). As such, a broad spectrum of both known and unidentified tumor antigens is presented to the immune system in the context of the potent immunostimulatory capacity of DCs. Murine studies have demonstrated the efficacy of fusion immunotherapy. However the clinical impact of DC/tumor fusion vaccines has been limited, suggesting that the immunosuppresive milieu found in patients with malignancies may blunt the efficacy of cancer vaccination. Thus, novel strategies to enhance fusion vaccine efficacy are needed. Regulatory T cells (Tregs) are known to suppress anti-tumor immunity, and depletion or functional inactivation of these cells improves immunotherapy in both animal models and clinical trials. In this study, we sought to investigate whether functional inactivation of CD4+CD25+FoxP3+ Treg with anti-CD25 monoclonal antibody (mAb) PC61 prior to DC/tumor vaccination would significantly improve immunotherapy in the murine B16 melanoma model.
Methods: Treg blockade was achieved with systemic PC61 administration. This blockage was done in conjunction with DC/tumor fusion vaccine administration to treat established melanoma pulmonary metastases. Enumeration of these metastases was performed and compared between experimental groups using Wilcoxon Rank Sum Test. IFN-gamma ELISPOT assay was performed on splenocytes from treated mice.
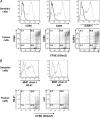
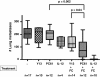
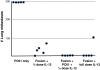
Results: We demonstrate that treatment of mice with established disease using mAb PC61 and DC/tumor fusion significantly reduced counts of pulmonary metastases compared to treatment with PC61 alone (p=0.002) or treatment with control antibody plus fusion vaccine (p=0.0397). Furthermore, IFN-gamma ELISPOT analyses reveal that the increase in cancer immunity was mediated by anti-tumor specific CD4+ T-helper cells, without concomitant induction of CD8+ cytotoxic T cells. Lastly, our data provide proof of principle that combination treatment with mAb PC61 and systemic IL-12 can lower the dose of IL-12 necessary to obtain maximal therapeutic efficacy.
Conclusions: To our knowledge, this is the first report investigating the effects of anti-CD25 mAb administration on DC/tumor-fusion vaccine efficacy in a murine melanoma model, and our results may aide the design of future clinical trials with enhanced therapeutic impact.
Figures

Similar articles
-
Local secretion of IL-12 augments the therapeutic impact of dendritic cell-tumor cell fusion vaccination.J Surg Res. 2013 Dec;185(2):904-11. doi: 10.1016/j.jss.2013.06.045. Epub 2013 Jul 17. J Surg Res. 2013. PMID: 23891424
-
Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer.Oncol Rep. 2009 Aug;22(2):337-43. Oncol Rep. 2009. PMID: 19578774
-
Administration of anti-CD25 mAb leads to impaired α-galactosylceramide-mediated induction of IFN-γ production in a murine model.Immunobiology. 2013 Jun;218(6):851-9. doi: 10.1016/j.imbio.2012.10.012. Epub 2012 Oct 26. Immunobiology. 2013. PMID: 23182710
-
Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells.Ann N Y Acad Sci. 2009 Sep;1174:99-106. doi: 10.1111/j.1749-6632.2009.04939.x. Ann N Y Acad Sci. 2009. PMID: 19769742 Review.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
Dendritic-Tumor Fusion Cell-Based Cancer Vaccines.Int J Mol Sci. 2016 May 26;17(6):828. doi: 10.3390/ijms17060828. Int J Mol Sci. 2016. PMID: 27240347 Free PMC article. Review.
-
Developing Effective Cancer Vaccines Using Rendered-Inactive Tumor Cells.Vaccines (Basel). 2023 Aug 5;11(8):1330. doi: 10.3390/vaccines11081330. Vaccines (Basel). 2023. PMID: 37631898 Free PMC article.
-
Targeting regulatory T cells to improve vaccine immunogenicity in early life.Front Microbiol. 2014 Sep 11;5:477. doi: 10.3389/fmicb.2014.00477. eCollection 2014. Front Microbiol. 2014. PMID: 25309517 Free PMC article. Review.
-
Regulatory T cells in the immunotherapy of melanoma.Tumour Biol. 2016 Jan;37(1):77-85. doi: 10.1007/s13277-015-4315-0. Epub 2015 Oct 30. Tumour Biol. 2016. PMID: 26515336 Review.
-
Protective effect of Flt3L on organ structure during advanced multiorgan dysfunction syndrome in mice.Mol Med Rep. 2015 Jun;11(6):4135-41. doi: 10.3892/mmr.2015.3328. Epub 2015 Feb 10. Mol Med Rep. 2015. PMID: 25672780 Free PMC article.
References
-
- Kuriyama H, Shimizu K, Lee W, Kjaergaard J, Parkhurst MR, Cohen PA. et al.Therapeutic vaccine generated by electrofusion of dendritic cells and tumour cells. Dev Biol Basel. 2004;116:169–78. discussion 79–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

