Interactions between a receptor tyrosine phosphatase and a cell surface ligand regulate axon guidance and glial-neuronal communication
- PMID: 23764287
- PMCID: PMC3683152
- DOI: 10.1016/j.neuron.2013.04.001
Interactions between a receptor tyrosine phosphatase and a cell surface ligand regulate axon guidance and glial-neuronal communication
Abstract
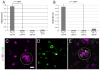
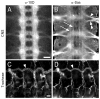
We developed a screening method for orphan receptor ligands, in which cell-surface proteins are expressed in Drosophila embryos from GAL4-dependent insertion lines and ligand candidates identified by the presence of ectopic staining with receptor fusion proteins. Stranded at second (Sas) binds to the receptor tyrosine phosphatase Ptp10D in embryos and in vitro. Sas and Ptp10D can interact in trans when expressed in cultured cells. Interactions between Sas and Ptp10D on longitudinal axons are required to prevent them from abnormally crossing the midline. Sas is expressed on both neurons and glia, whereas Ptp10D is restricted to CNS axons. We conducted epistasis experiments by overexpressing Sas in glia and examining how the resulting phenotypes are changed by removal of Ptp10D from neurons. We find that neuronal Ptp10D restrains signaling by overexpressed glial Sas, which would otherwise produce strong glial and axonal phenotypes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Redundancy and compensation in axon guidance: genetic analysis of the Drosophila Ptp10D/Ptp4E receptor tyrosine phosphatase subfamily.Neural Dev. 2008 Jan 31;3:3. doi: 10.1186/1749-8104-3-3. Neural Dev. 2008. PMID: 18237413 Free PMC article.
-
Differential ligand regulation of PlexB signaling in motor neuron axon guidance in Drosophila.Int J Dev Neurosci. 2016 Dec;55:34-40. doi: 10.1016/j.ijdevneu.2016.09.006. Epub 2016 Sep 13. Int J Dev Neurosci. 2016. PMID: 27637927
-
A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS.Neuron. 2011 Apr 28;70(2):281-98. doi: 10.1016/j.neuron.2011.02.050. Neuron. 2011. PMID: 21521614 Free PMC article.
-
Coupling glial numbers and axonal patterns.Cell Cycle. 2004 Sep;3(9):1118-20. Epub 2004 Sep 15. Cell Cycle. 2004. PMID: 15326396 Review.
-
Keeping the CNS clear: glial phagocytic functions in Drosophila.Glia. 2011 Sep;59(9):1304-11. doi: 10.1002/glia.21098. Epub 2010 Dec 6. Glia. 2011. PMID: 21136555 Review.
Cited by
-
RasV12; scrib-/- Tumors: A Cooperative Oncogenesis Model Fueled by Tumor/Host Interactions.Int J Mol Sci. 2021 Aug 18;22(16):8873. doi: 10.3390/ijms22168873. Int J Mol Sci. 2021. PMID: 34445578 Free PMC article. Review.
-
Visualization of binding patterns for five Leucine-rich repeat proteins in the Drosophila embryo.MicroPubl Biol. 2019 Dec 18;2019:10.17912/micropub.biology.000199. doi: 10.17912/micropub.biology.000199. MicroPubl Biol. 2019. PMID: 32550403 Free PMC article. No abstract available.
-
Cadherin 99C regulates apical expansion and cell rearrangement during epithelial tube elongation.Development. 2014 May;141(9):1950-60. doi: 10.1242/dev.104166. Epub 2014 Apr 9. Development. 2014. PMID: 24718992 Free PMC article.
-
Elimination of oncogenic cells that regulate epithelial homeostasis in Drosophila.Dev Growth Differ. 2019 Jun;61(5):337-342. doi: 10.1111/dgd.12604. Epub 2019 Apr 7. Dev Growth Differ. 2019. PMID: 30957223 Free PMC article. Review.
-
Transsynaptic interactions between IgSF proteins DIP-α and Dpr10 are required for motor neuron targeting specificity.Elife. 2019 Feb 4;8:e42690. doi: 10.7554/eLife.42690. Elife. 2019. PMID: 30714906 Free PMC article.
References
-
- Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, van der Merwe PA, Jones EY. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–1220. - PubMed
-
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. - PubMed
-
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

