Akt-signal integration is involved in the differentiation of embryonal carcinoma cells
- PMID: 23762260
- PMCID: PMC3675137
- DOI: 10.1371/journal.pone.0064877
Akt-signal integration is involved in the differentiation of embryonal carcinoma cells
Abstract
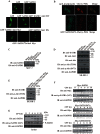
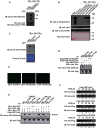
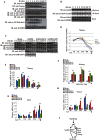
The mechanism by which Akt modulates stem cell homeostasis is still incompletely defined. Here we demonstrate that Akt phosphorylates special AT-rich sequences binding protein 1 (SATB1) at serine 47 and protects SATB1 from apoptotic cleavage. Meanwhile, Akt phosphorylates Oct4 at threonine 228 and Klf4 at threonine 399, and accelerates their degradation. Moreover, PI3K/Akt signaling enhances the binding of SATB1 to Sox2, thereby probably impairing the formation of Oct4/Sox2 regulatory complexes. During retinoic acid (RA)-induced differentiation of mouse F9 embryonal carcinoma cells (ECCs), the Akt activation profile as well as its substrate spectrum is strikingly correlated with the down-regulation of Oct4, Klf4 and Nanog, which suggests Akt activation is coupled to the onset of differentiation. Accordingly, Akt-mediated phosphorylation is crucial for the capability of SATB1 to repress Nanog expression and to activate transcription of Bcl2 and Nestin genes. Taken together, we conclude that Akt is involved in the differentiation of ECCs through coordinated phosphorylations of pluripotency/differentiation factors.
Conflict of interest statement
Figures



Similar articles
-
Wnt/β-catenin signaling pathway upregulates c-Myc expression to promote cell proliferation of P19 teratocarcinoma cells.Anat Rec (Hoboken). 2012 Dec;295(12):2104-13. doi: 10.1002/ar.22592. Epub 2012 Sep 14. Anat Rec (Hoboken). 2012. PMID: 22976998
-
Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells.Mol Cell. 2012 Nov 30;48(4):627-40. doi: 10.1016/j.molcel.2012.08.030. Epub 2012 Oct 4. Mol Cell. 2012. PMID: 23041284 Free PMC article.
-
Stable expression of FoxA1 promotes pluripotent P19 embryonal carcinoma cells to be neural stem-like cells.Gene Expr. 2012;15(4):153-62. doi: 10.3727/105221612x13372578119571. Gene Expr. 2012. PMID: 22783724 Free PMC article.
-
Crosstalks between Raf-kinase inhibitor protein and cancer stem cell transcription factors (Oct4, KLF4, Sox2, Nanog).Tumour Biol. 2017 Apr;39(4):1010428317692253. doi: 10.1177/1010428317692253. Tumour Biol. 2017. PMID: 28378634 Review.
-
Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs.Stem Cells Dev. 2009 Sep;18(7):1093-108. doi: 10.1089/scd.2009.0113. Stem Cells Dev. 2009. PMID: 19480567 Free PMC article. Review.
Cited by
-
Reduced Satb1 expression predisposes CD4+ T conventional cells to Treg suppression and promotes transplant survival.Proc Natl Acad Sci U S A. 2022 Oct 4;119(40):e2205062119. doi: 10.1073/pnas.2205062119. Epub 2022 Sep 26. Proc Natl Acad Sci U S A. 2022. PMID: 36161903 Free PMC article.
-
SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells.Onco Targets Ther. 2018 Oct 11;11:6811-6825. doi: 10.2147/OTT.S182437. eCollection 2018. Onco Targets Ther. 2018. PMID: 30349314 Free PMC article.
-
The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review.Int J Stem Cells. 2020 Nov 30;13(3):312-325. doi: 10.15283/ijsc20097. Int J Stem Cells. 2020. PMID: 32840233 Free PMC article. Review.
-
Modulation of mitochondrial respiration underpins neuronal differentiation enhanced by lutein.Neural Regen Res. 2019 Jan;14(1):87-99. doi: 10.4103/1673-5374.243713. Neural Regen Res. 2019. PMID: 30531082 Free PMC article.
-
AKT-driven phospho-patterns of pluripotency.Cell Cycle. 2015;14(24):3784-5. doi: 10.1080/15384101.2015.1115642. Cell Cycle. 2015. PMID: 26697835 Free PMC article. No abstract available.
References
-
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, et al. (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441: 475–482. - PubMed
-
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, et al. (2006) PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441: 518–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

