Adaptation to seasonality and the winter freeze
- PMID: 23761798
- PMCID: PMC3669742
- DOI: 10.3389/fpls.2013.00167
Adaptation to seasonality and the winter freeze
Abstract
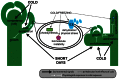
Flowering plants initially diversified during the Mesozoic era at least 140 million years ago in regions of the world where temperate seasonal environments were not encountered. Since then several cooling events resulted in the contraction of warm and wet environments and the establishment of novel temperate zones in both hemispheres. In response, less than half of modern angiosperm families have members that evolved specific adaptations to cold seasonal climates, including cold acclimation, freezing tolerance, endodormancy, and vernalization responsiveness. Despite compelling evidence for multiple independent origins, the level of genetic constraint on the evolution of adaptations to seasonal cold is not well understood. However, the recent increase in molecular genetic studies examining the response of model and crop species to seasonal cold offers new insight into the evolutionary lability of these traits. This insight has major implications for our understanding of complex trait evolution, and the potential role of local adaptation in response to past and future climate change. In this review, we discuss the biochemical, morphological, and developmental basis of adaptations to seasonal cold, and synthesize recent literature on the genetic basis of these traits in a phylogenomic context. We find evidence for multiple genetic links between distinct physiological responses to cold, possibly reinforcing the coordinated expression of these traits. Furthermore, repeated recruitment of the same or similar ancestral pathways suggests that land plants might be somewhat pre-adapted to dealing with temperature stress, perhaps making inducible cold traits relatively easy to evolve.
Keywords: cold acclimation; endodormancy; freezing tolerance; plant adaptation; seasonality; vernalization responsiveness.
Figures


Similar articles
-
Evolution of Cold Acclimation and Its Role in Niche Transition in the Temperate Grass Subfamily Pooideae.Plant Physiol. 2019 May;180(1):404-419. doi: 10.1104/pp.18.01448. Epub 2019 Mar 8. Plant Physiol. 2019. PMID: 30850470 Free PMC article.
-
Successive evolutionary steps drove Pooideae grasses from tropical to temperate regions.New Phytol. 2018 Jan;217(2):925-938. doi: 10.1111/nph.14868. Epub 2017 Nov 1. New Phytol. 2018. PMID: 29091285
-
Treatment Analogous to Seasonal Change Demonstrates the Integration of Cold Responses in Brachypodium distachyon.Plant Physiol. 2020 Feb;182(2):1022-1038. doi: 10.1104/pp.19.01195. Epub 2019 Dec 16. Plant Physiol. 2020. PMID: 31843801 Free PMC article.
-
The interaction between freezing tolerance and phenology in temperate deciduous trees.Front Plant Sci. 2014 Oct 10;5:541. doi: 10.3389/fpls.2014.00541. eCollection 2014. Front Plant Sci. 2014. PMID: 25346748 Free PMC article. Review.
-
Biochemical adaptations of notothenioid fishes: comparisons between cold temperate South American and New Zealand species and Antarctic species.Comp Biochem Physiol A Mol Integr Physiol. 2007 Jul;147(3):799-807. doi: 10.1016/j.cbpa.2006.09.028. Epub 2006 Dec 5. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 17293146 Review.
Cited by
-
Annotation of Siberian Larch (Larix sibirica Ledeb.) Nuclear Genome-One of the Most Cold-Resistant Tree Species in the Only Deciduous GENUS in Pinaceae.Plants (Basel). 2022 Aug 6;11(15):2062. doi: 10.3390/plants11152062. Plants (Basel). 2022. PMID: 35956540 Free PMC article.
-
Understanding Past, and Predicting Future, Niche Transitions based on Grass Flowering Time Variation.Plant Physiol. 2020 Jul;183(3):822-839. doi: 10.1104/pp.20.00100. Epub 2020 May 13. Plant Physiol. 2020. PMID: 32404414 Free PMC article. Review.
-
Sexual modulation in a polyploid grass: a reproductive contest between environmentally inducible sexual and genetically dominant apomictic pathways.Sci Rep. 2020 May 20;10(1):8319. doi: 10.1038/s41598-020-64982-6. Sci Rep. 2020. PMID: 32433575 Free PMC article.
-
Chilling acclimation provides immunity to stress by altering regulatory networks and inducing genes with protective functions in cassava.BMC Plant Biol. 2014 Aug 5;14:207. doi: 10.1186/s12870-014-0207-5. BMC Plant Biol. 2014. PMID: 25090992 Free PMC article.
-
Expansion of the phosphatidylethanolamine binding protein family in legumes: a case study of Lupinus angustifolius L. FLOWERING LOCUS T homologs, LanFTc1 and LanFTc2.BMC Genomics. 2016 Oct 21;17(1):820. doi: 10.1186/s12864-016-3150-z. BMC Genomics. 2016. PMID: 27769166 Free PMC article.
References
-
- Adhikari K. N., Buirchell B. J., Sweetingham M. W. (2012). Length of vernalization period affects flowering time in three lupin species. Plant Breed. 131 631–636 10.1111/j.1439-0523.2012.01996.x - DOI
-
- Agarwal P. K., Jha B. (2010). Transcription factors in plants and ABA dependent and independent abiotic stress signaling. Biol. Plant. 54 201–212 10.1007/s10535-010-0038-7 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

