Cyclic nucleotide permeability through unopposed connexin hemichannels
- PMID: 23760880
- PMCID: PMC3674318
- DOI: 10.3389/fphar.2013.00075
Cyclic nucleotide permeability through unopposed connexin hemichannels
Abstract
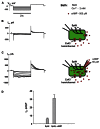
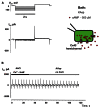
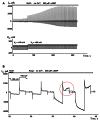
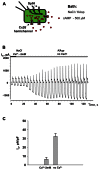
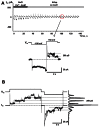
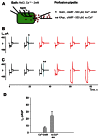
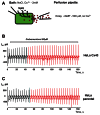
Cyclic adenosine monophosphate (cAMP) is a well-known intracellular and intercellular second messenger. The membrane permeability of such molecules has potential importance for autocrine-like or paracrine-like delivery. Here experiments have been designed to demonstrate whether gap junction hemichannels, composed of connexins, are a possible entrance pathway for cyclic nucleotides into the interior of cells. HeLa cells stably expressing connexin43 (Cx43) and connexin26 (Cx26) were used to study the cyclic nucleotide permeability of gap junction hemichannels. For the detection of cAMP uptake, the cells were transfected using the cyclic nucleotide-modulated channel from sea urchin sperm (SpIH) as the cAMP sensor. SpIH derived currents (I m) were recorded in whole-cell/perforated patch clamp configuration. Perfusion of the cells in an external K(+) aspartate(-) (KAsp) solution containing 500 μM cAMP and no extracellular Ca(2) (+), yielded a five to sevenfold increase in the I m current level. The SpIH current increase was associated with detectable hemichannel current activity. Depolarization of cells in Ca(2) (+)-free NaCl perfusate with 500 μM cAMP also induced a SpIH current increase. Elevating extracellular Ca(2) (+) to mM levels inhibited hemichannel activity. Perfusion with a depolarizing KAsp solution containing 500 μM cAMP and 2 mM Ca(2) (+) did not increase SpIH currents. The addition of the gap junction blocker carbenoxolone to the external solution inhibited cAMP uptake. Both cell depolarization and lowered extracellular Ca(2) (+) increase the open probability of non-junctional hemichannels. Accordingly, the SpIH current augmentation was induced by the uptake of extracellular cAMP via open membrane hemichannels in Cx43 and Cx26 expressing cells. The data presented here show that hemichannels of Cx43 and Cx26 are permeable to cAMP, and further the data suggest that hemichannels are, in fact, a potential pathway for cAMP mediated cell-to-cell signaling.
Keywords: connexin26; connexin43; cyclic AMP; electrophysiology; gap junction; permeability.
Figures


Similar articles
-
Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides.J Gen Physiol. 2008 Apr;131(4):293-305. doi: 10.1085/jgp.200709934. J Gen Physiol. 2008. PMID: 18378798 Free PMC article.
-
Lens Connexin Channels Have Differential Permeability to the Second Messenger cAMP.Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):3821-3829. doi: 10.1167/iovs.19-27302. Invest Ophthalmol Vis Sci. 2019. PMID: 31529078 Free PMC article.
-
Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells.J Gen Physiol. 2002 Feb;119(2):147-64. doi: 10.1085/jgp.119.2.147. J Gen Physiol. 2002. PMID: 11815665 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Gap junction hemichannels in astrocytes of the CNS.Acta Physiol Scand. 2003 Sep;179(1):9-22. doi: 10.1046/j.1365-201X.2003.01196.x. Acta Physiol Scand. 2003. PMID: 12940934 Free PMC article. Review.
Cited by
-
Connexins form functional hemichannels in porcine ciliary epithelium.Exp Eye Res. 2014 Jan;118:20-9. doi: 10.1016/j.exer.2013.11.004. Epub 2013 Nov 19. Exp Eye Res. 2014. PMID: 24262135 Free PMC article.
-
A novel GJA8 mutation (p.V44A) causing autosomal dominant congenital cataract.PLoS One. 2014 Dec 17;9(12):e115406. doi: 10.1371/journal.pone.0115406. eCollection 2014. PLoS One. 2014. PMID: 25517998 Free PMC article.
-
Measuring Connexin Hemichannel Opening in Response to an InsP3-Mediated Cytosolic Ca2+ Increase.Methods Mol Biol. 2024;2801:189-197. doi: 10.1007/978-1-0716-3842-2_14. Methods Mol Biol. 2024. PMID: 38578422
-
cGAMP the travelling messenger.Front Immunol. 2023 May 23;14:1150705. doi: 10.3389/fimmu.2023.1150705. eCollection 2023. Front Immunol. 2023. PMID: 37287967 Free PMC article. Review.
-
A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators.Int J Mol Sci. 2021 Jan 31;22(3):1417. doi: 10.3390/ijms22031417. Int J Mol Sci. 2021. PMID: 33572565 Free PMC article.
References
-
- Anselmi F., Hernandez V. H., Crispino G., Seydel A., Ortolano S., Roper S. D., et al. (2008). ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. U.S.A. 105 18770–18775 10.1073/pnas.0800793105 - DOI - PMC - PubMed
-
- Bukauskas F. F., Jordan K., Bukauskiene A., Bennett M. V., Lampe P. D., Laird D. W., et al. (2000). Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U.S.A. 97 2556–2561 10.1073/pnas.050588497 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

