Role of antigen-specific regulatory CD4+CD25+ T cells in tolerance induction after neonatal IP administration of AAV-hF.IX
- PMID: 23759700
- PMCID: PMC3795474
- DOI: 10.1038/gt.2013.22
Role of antigen-specific regulatory CD4+CD25+ T cells in tolerance induction after neonatal IP administration of AAV-hF.IX
Abstract
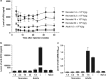
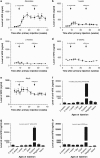
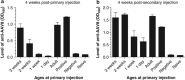
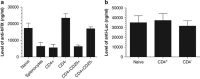
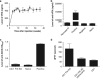
Neonatal AAV8-mediated Factor IX (F.IX) gene delivery was applied as a model for exploring mechanisms of tolerance induction during immune ontogeny. Intraperitoneal delivery of AAV8/ Factor IX (hF.IX) during weeks 1-4 of life, over a 20-fold dose range, directed stable hF.IX expression, correction of coagulopathy in F.IX-null hemophilia B mice, and induction of tolerance to hF.IX; however, only primary injection at 1-2 days of life enabled increasing AAV8-mediated hF.IX expression after re-administration, due to the absence of anti-viral capsid antibodies. Adoptive splenocyte transfer from tolerized mice demonstrated induction of CD4(+)CD25(+) T regulatory (T(reg)) populations that specifically suppressed anti-hF.IX antibody responses, but not responses to third party antigen. Induction of hF.IX antibodies was only observed in tolerized mice after in vivo CD4(+)CD25(+) cell depletion and hF.IX challenge. Thus, primary injection of AAV during a critical period in the first week of life does not elicit antiviral responses, enabling re-administration of AAV and augmentation of hF.IX levels. Expansion of hF.IX-specific CD4(+)CD25(+) T(regs) has a major role in tolerance induction early in immune ontogeny. Neonatal gene transfer provides a useful approach for defining the ontogeny of immune responses and may suggest approaches for inducing tolerance in the context of genetic therapies.
Figures
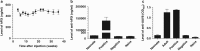


Similar articles
-
Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice.Mol Ther. 2007 Sep;15(9):1677-85. doi: 10.1038/sj.mt.6300219. Epub 2007 Jun 12. Mol Ther. 2007. PMID: 17565352
-
Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer.Hum Gene Ther. 2009 Jul;20(7):767-76. doi: 10.1089/hum.2008.161. Hum Gene Ther. 2009. PMID: 19309290 Free PMC article.
-
Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer.Blood. 2007 Aug 15;110(4):1132-40. doi: 10.1182/blood-2007-02-073304. Epub 2007 Apr 16. Blood. 2007. PMID: 17438084 Free PMC article.
-
Complexity of immune responses to AAV transgene products - Example of factor IX.Cell Immunol. 2019 Aug;342:103658. doi: 10.1016/j.cellimm.2017.05.006. Epub 2017 May 29. Cell Immunol. 2019. PMID: 28645365 Free PMC article. Review.
-
Technology evaluation: AAV factor IX gene therapy, Avigen Inc.Curr Opin Mol Ther. 2000 Oct;2(5):601-6. Curr Opin Mol Ther. 2000. PMID: 11249763 Review.
Cited by
-
Production of lentiviral vectors using novel, enzymatically produced, linear DNA.Gene Ther. 2019 Apr;26(3-4):86-92. doi: 10.1038/s41434-018-0056-1. Epub 2019 Jan 14. Gene Ther. 2019. PMID: 30643205 Free PMC article.
-
Argininosuccinic aciduria fosters neuronal nitrosative stress reversed by Asl gene transfer.Nat Commun. 2018 Aug 29;9(1):3505. doi: 10.1038/s41467-018-05972-1. Nat Commun. 2018. PMID: 30158522 Free PMC article.
-
Neonatal Gene Therapy for Hemophilia B by a Novel Adenovirus Vector Showing Reduced Leaky Expression of Viral Genes.Mol Ther Methods Clin Dev. 2017 Jul 8;6:183-193. doi: 10.1016/j.omtm.2017.07.001. eCollection 2017 Sep 15. Mol Ther Methods Clin Dev. 2017. PMID: 28828393 Free PMC article.
-
Gene therapy for monogenic liver diseases: clinical successes, current challenges and future prospects.J Inherit Metab Dis. 2017 Jul;40(4):497-517. doi: 10.1007/s10545-017-0053-3. Epub 2017 May 31. J Inherit Metab Dis. 2017. PMID: 28567541 Free PMC article. Review.
-
Induction of Immune Tolerance to Foreign Protein via Adeno-Associated Viral Vector Gene Transfer in Mid-Gestation Fetal Sheep.PLoS One. 2017 Jan 31;12(1):e0171132. doi: 10.1371/journal.pone.0171132. eCollection 2017. PLoS One. 2017. PMID: 28141818 Free PMC article.
References
-
- Mannucci PM, Tuddenbam EG. The hemophilias: progress and problems. Semin Hematol. 1999;36:104–117. - PubMed
-
- High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9 (Suppl 1:2–11. - PubMed
-
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. - PubMed
-
- McCarty DM, Young SM, Jr, Samulski RJ. Integration of Adeno-Associated Virus (AAV) and Recombinant AAV Vectors. Annu Rev Genet. 2004;38:819–845. - PubMed
-
- Stilwell JL, Samulski RJ.Adeno-associated virus vectors for therapeutic gene transfer Biotechniques 200334148–150.152, 154 passim). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

