Laminin drives survival signals to promote a contractile smooth muscle phenotype and airway hyperreactivity
- PMID: 23756649
- PMCID: PMC6159668
- DOI: 10.1096/fj.12-221341
Laminin drives survival signals to promote a contractile smooth muscle phenotype and airway hyperreactivity
Abstract
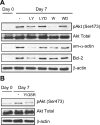
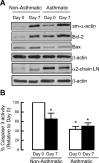
Increased airway smooth muscle (ASM) mass is believed to underlie the relatively fixed airway hyperresponsiveness (AHR) in asthma. Developments of therapeutic approaches to reverse airway remodeling are impeded by our lack of insight on the mechanisms behind the increase in mass of contractile ASM cells. Increased expression of laminin, an extracellular matrix protein, is associated with asthma. Our studies investigate the role of laminin-induced ASM survival signals in the development of increased ASM and AHR. Antagonizing laminin integrin binding using the laminin-selective competing peptide, YIGSR, and mimicking laminin with exogenous α2-chain laminin, we show that laminin is both necessary and sufficient to induce ASM cell survival, concomitant with the induction of ASM contractile phenotype. Using siRNA, we show that the laminin-binding integrin α7β1 mediates this process. Moreover, in laminin-211-deficient mice, allergen-induced AHR was not observed. Notably, ASM cells from asthmatic airways express a higher abundance of intracellular cell survival proteins, consistent with a role for reduced rates of cell apoptosis in development of ASM hyperplasia. Targeting the laminin-integrin α7β1 signaling pathway may offer new avenues for the development of therapies to reduce the increase in mass of contractile phenotype ASM cells that underlie AHR in asthma.
Keywords: apoptosis; integrin; phenotype plasticity.
Conflict of interest statement
The authors thank Ms. Karol McNeill and Ms. Tze Khee Chan (TUNEL assay) for excellent technical assistance.
Figures


Similar articles
-
Integrin α7 expression is increased in asthmatic patients and its inhibition reduces Kras protein abundance in airway smooth muscle cells.Sci Rep. 2019 Jul 9;9(1):9892. doi: 10.1038/s41598-019-46260-2. Sci Rep. 2019. PMID: 31289310 Free PMC article.
-
The laminin β1-competing peptide YIGSR induces a hypercontractile, hypoproliferative airway smooth muscle phenotype in an animal model of allergic asthma.Respir Res. 2010 Dec 3;11(1):170. doi: 10.1186/1465-9921-11-170. Respir Res. 2010. PMID: 21129174 Free PMC article.
-
Laminin α4 contributes to airway remodeling and inflammation in asthma.Am J Physiol Lung Cell Mol Physiol. 2019 Dec 1;317(6):L768-L777. doi: 10.1152/ajplung.00222.2019. Epub 2019 Sep 25. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31553662
-
The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease.Eur Respir J. 2000 Aug;16(2):349-54. doi: 10.1034/j.1399-3003.2000.16b25.x. Eur Respir J. 2000. PMID: 10968513 Review.
-
Tissue and matrix influences on airway smooth muscle function.Pulm Pharmacol Ther. 2009 Oct;22(5):379-87. doi: 10.1016/j.pupt.2008.12.007. Epub 2008 Dec 24. Pulm Pharmacol Ther. 2009. PMID: 19135163 Review.
Cited by
-
Plk1 in Asthma: Ready for Primetime?Am J Respir Cell Mol Biol. 2022 Feb;66(2):124-125. doi: 10.1165/rcmb.2021-0425ED. Am J Respir Cell Mol Biol. 2022. PMID: 34748723 Free PMC article. No abstract available.
-
Airway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L912-33. doi: 10.1152/ajplung.00259.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24142517 Free PMC article. Review.
-
Integrin α7 expression is increased in asthmatic patients and its inhibition reduces Kras protein abundance in airway smooth muscle cells.Sci Rep. 2019 Jul 9;9(1):9892. doi: 10.1038/s41598-019-46260-2. Sci Rep. 2019. PMID: 31289310 Free PMC article.
-
Impact of CD151 overexpression on prognosis and therapy in non-small cell lung cancer patients lacking EGFR mutations.Cell Prolif. 2024 Sep;57(9):e13708. doi: 10.1111/cpr.13708. Epub 2024 Jul 9. Cell Prolif. 2024. PMID: 38982031 Free PMC article.
-
Transgenic overexpression of α7 integrin in smooth muscle attenuates allergen-induced airway inflammation in a murine model of asthma.FASEB Bioadv. 2022 Sep 12;4(11):724-740. doi: 10.1096/fba.2022-00050. eCollection 2022 Nov. FASEB Bioadv. 2022. PMID: 36349295 Free PMC article.
References
-
- Benayoun L., Druilhe A., Dombret M. C., Aubier M., Pretolani M. (2003) Airway structural alterations selectively associated with severe asthma. Am. J. Respir. Crit. Care Med. , 1360–1368 - PubMed
-
- Lambert R. K., Wiggs B. R., Kuwano K., Hogg J. C., Pare P. D. (1993) Functional significance of increased airway smooth muscle in asthma and COPD. J. Appl. Physiol. , 2771–2781 - PubMed
-
- Stewart A. G. (2004) Emigration and immigration of mesenchymal cells: a multicultural airway wall. Eur. Respir. J. , 515–517 - PubMed
-
- Ward J. E., Harris T., Bamford T., Mast A., Pain M. C., Robertson C., Smallwood D., Tran T., Wilson J., Stewart A. G. (2008) Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur. Respir. J. , 362–371 - PubMed
-
- Bentley J. K., Deng H., Linn M. J., Lei J., Dokshin G. A., Fingar D. C., Bitar K. N., Henderson W. R., Hershenson M. B. (2009) Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3(beta) phosphorylation in a mouse model of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. , L176–L184 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

