Heterogeneous effects of calorie restriction on in vivo glucose uptake and insulin signaling of individual rat skeletal muscles
- PMID: 23755179
- PMCID: PMC3670927
- DOI: 10.1371/journal.pone.0065118
Heterogeneous effects of calorie restriction on in vivo glucose uptake and insulin signaling of individual rat skeletal muscles
Abstract
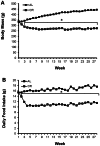
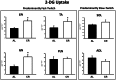
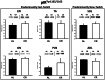
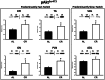
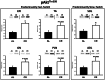
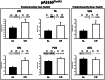
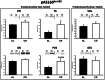
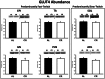
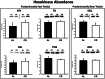
Calorie restriction (CR) (consuming ~60% of ad libitum, AL, intake) improves whole body insulin sensitivity and enhances insulin-stimulated glucose uptake by isolated skeletal muscles. However, little is known about CR-effects on in vivo glucose uptake and insulin signaling in muscle. Accordingly, 9-month-old male AL and CR (initiated when 3-months-old) Fischer 344 x Brown Norway rats were studied using a euglycemic-hyperinsulinemic clamp with plasma insulin elevated to a similar level (~140 µU/ml) in each diet group. Glucose uptake (assessed by infusion of [(14)C]-2-deoxyglucose, 2-DG), phosphorylation of key insulin signaling proteins (insulin receptor, Akt and Akt substrate of 160 kDa, AS160), abundance of GLUT4 and hexokinase proteins, and muscle fiber type composition (myosin heavy chain, MHC, isoform percentages) were determined in four predominantly fast-twitch (epitrochlearis, gastrocnemius, tibialis anterior, plantaris) and two predominantly slow-twitch (soleus, adductor longus) muscles. CR did not result in greater GLUT4 or hexokinase abundance in any of the muscles, and there were no significant diet-related effects on percentages of MHC isoforms. Glucose infusion was greater for CR versus AL rats (P<0.05) concomitant with significantly (P<0.05) elevated 2-DG uptake in 3 of the 4 fast-twitch muscles (epitrochlearis, gastrocnemius, tibialis anterior), without a significant diet-effect on 2-DG uptake by the plantaris or either slow-twitch muscle. Each of the muscles with a CR-related increase in 2-DG uptake was also characterized by significant (P<0.05) increases in phosphorylation of both Akt and AS160. Among the 3 muscles without a CR-related increase in glucose uptake, only the soleus had significant (P<0.05) CR-related increases in Akt and AS160 phosphorylation. The current data revealed that CR leads to greater whole body glucose disposal in part attributable to elevated in vivo insulin-stimulated glucose uptake by fast-twitch muscles. The results also demonstrated that CR does not uniformly enhance either insulin signaling or insulin-stimulated glucose uptake in all muscles in vivo.
Conflict of interest statement
Figures
Similar articles
-
Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats.J Gerontol A Biol Sci Med Sci. 2012 Dec;67(12):1279-85. doi: 10.1093/gerona/gls085. Epub 2012 Mar 27. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22454372 Free PMC article.
-
Akt substrate of 160 kDa is essential for the calorie restriction-induced increase in insulin-stimulated glucose uptake by skeletal muscle of female rats.Appl Physiol Nutr Metab. 2023 Mar 1;48(3):283-292. doi: 10.1139/apnm-2022-0414. Epub 2023 Jan 12. Appl Physiol Nutr Metab. 2023. PMID: 36634338
-
Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model.PLoS One. 2019 Apr 29;14(4):e0216236. doi: 10.1371/journal.pone.0216236. eCollection 2019. PLoS One. 2019. PMID: 31034517 Free PMC article.
-
Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
-
AKT ISOFORMS-AS160-GLUT4: The defining axis of insulin resistance.Rev Endocr Metab Disord. 2021 Dec;22(4):973-986. doi: 10.1007/s11154-021-09652-2. Epub 2021 Apr 30. Rev Endocr Metab Disord. 2021. PMID: 33928491 Review.
Cited by
-
Greater Phosphorylation of AMPK and Multiple AMPK Substrates in the Skeletal Muscle of 24-Month-Old Calorie Restricted Compared to Ad-Libitum Fed Male Rats.J Gerontol A Biol Sci Med Sci. 2023 Feb 24;78(2):177-185. doi: 10.1093/gerona/glac218. J Gerontol A Biol Sci Med Sci. 2023. PMID: 36269629 Free PMC article.
-
Wushenziye Formula Improves Skeletal Muscle Insulin Resistance in Type 2 Diabetes Mellitus via PTP1B-IRS1-Akt-GLUT4 Signaling Pathway.Evid Based Complement Alternat Med. 2017;2017:4393529. doi: 10.1155/2017/4393529. Epub 2017 Dec 31. Evid Based Complement Alternat Med. 2017. PMID: 29479370 Free PMC article.
-
Inhibition of Akt2 phosphorylation abolishes the calorie restriction-induced improvement in insulin-stimulated glucose uptake by rat soleus muscle.Appl Physiol Nutr Metab. 2016 Nov;41(11):1208-1211. doi: 10.1139/apnm-2016-0326. Epub 2016 Aug 16. Appl Physiol Nutr Metab. 2016. PMID: 27786542 Free PMC article.
-
Calorie restriction leads to greater Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle from old rats.Am J Physiol Regul Integr Comp Physiol. 2016 Mar 1;310(5):R449-58. doi: 10.1152/ajpregu.00449.2015. Epub 2016 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26739650 Free PMC article.
-
Intracerebroventricular Injection of Alarin Increased Glucose Uptake in Skeletal Muscle of Diabetic Rats.PLoS One. 2015 Oct 6;10(10):e0139327. doi: 10.1371/journal.pone.0139327. eCollection 2015. PLoS One. 2015. PMID: 26439383 Free PMC article.
References
-
- Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM (1999) Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 86: 1930–1935. - PubMed
-
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, et al. (1994) Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 17: 30–36. - PubMed
-
- Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, et al. (1994) Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol 266: E540–547. - PubMed
-
- Lawler DF, Larson BT, Ballam JM, Smith GK, Biery DN, et al. (2008) Diet restriction and ageing in the dog: major observations over two decades. Br J Nutr 99: 793–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

